| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:43:58 UTC |
|---|
| Update Date | 2016-11-09 01:15:28 UTC |
|---|
| Accession Number | CHEM016797 |
|---|
| Identification |
|---|
| Common Name | Naloxone |
|---|
| Class | Small Molecule |
|---|
| Description | Naloxone is an opioid antagonist medication used to block or reverse the effects of opioid drugs, particularly within the setting of drug overdoses which are rapidly becoming a leading cause of death worldwide. More specifically, naloxone has a high affinity for μ-opioid receptors, where it acts as an inverse agonist, causing the rapid removal of any other drugs bound to these receptors. When taken in large quantities, opioid medications such as , , , , or are capable of causing life-threatening symptoms such as respiratory depression, reduced heart rate, slurred speech, drowsiness, and constricted pupils. If untreated, this can progress to vomiting, absent pulse and breathing, loss of consciousness, and even death. Naloxone is indicated for the rapid reversal of these symptoms of central nervous system depression in opioid overdose. It's important to note that naloxone only works on opioid receptors within the body, and is therefore not capable of reversing the effects of non-opioid medications such as stimulants like or , or benzodiazepines like or .
Also known as the brand name product Narcan, naloxone is currently available by intramuscular (IM) or subcutaneous (SubQ) injection, nasal spray, or intravenous (IV) infusion. Naloxone IM injections are commonly available in the form of "kits", which is ideal for making overdose treatment accessible and readily available for administration by minimally trained individuals within the community. Kits commonly include the supplies necessary to treat an overdose in a non-medical setting such as alcohol swabs, syringes, a rescue breathing mask, and instructions for use. Frequently also carried by medical and emergency personnel and at events known to be associated with heavy drug use like music festivals, naloxone kits are considered a key component of harm reduction strategies.
When injected intramuscularly (IM), naloxone acts within 3-5 minutes. Administration of naloxone is associated with very few side effects. Notably, if injected into a person not currently using opioid medications, there would be no noticeable effects at all. However, for individuals using opioid medications or experiencing an overdose, IM injection of naloxone rapidly reverses opioid effects and can cause the injected individual to immediately experience withdrawal symptoms. Common symptoms of opioid withdrawal include nausea, vomiting, sweating, runny nose, aches, and diarrhea. Although certainly physically uncomfortable, opioid withdrawal symptoms are not life-threatening; administration of naloxone is therefore appropriate for any person appearing to have any symptoms of an opioid overdose. Due to its short duration of action, persons injected with naloxone should be monitored for responsiveness and potentially injected a second time or taken to the hospital.
Naloxone is also available within the combination product Suboxone with the opioid medication . Suboxone is used for the maintenance treatment of opioid dependence and addiction. When taken orally, naloxone has no pharmacological effect and does not reduce the effectiveness of the opioid effect of buprenorphine. The primary purpose of including naloxone within Suboxone is to act as a deterrent to injection, as injected naloxone would rapidly reverse the effects of buprenorphine. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-Naloxone | ChEBI | | 1-N-Allyl-14-hydroxynordihydromorphinone | ChEBI | | 17-Allyl-3,14-dihydroxy-4,5alpha-epoxymorphinan-6-one | ChEBI | | Naloxona | ChEBI | | Naloxonum | ChEBI | | DBL Naloxone | Kegg | | 17-Allyl-3,14-dihydroxy-4,5a-epoxymorphinan-6-one | Generator | | 17-Allyl-3,14-dihydroxy-4,5α-epoxymorphinan-6-one | Generator | | EN 1530 base | HMDB | | L-Naloxone | HMDB | | N-Allylnoroxymorphone | HMDB | | Nalossone | HMDB | | Abello brand OF naloxone hydrochloride | HMDB | | Abello, naloxone | HMDB | | Boots brand OF naloxone hydrochloride | HMDB | | Bristol-myers squibb brand OF naloxone hydrochloride | HMDB | | Curamed brand OF naloxone hydrochloride | HMDB | | endo Brand OF naloxone hydrochloride | HMDB | | Narcanti | HMDB | | Ratiopharm brand OF naloxone hydrochloride | HMDB | | Dihydride, naloxone hydrochloride | HMDB | | Naloxon ratiopharm | HMDB | | Naloxone hydrochloride | HMDB | | Naloxone hydrochloride, (5 beta,9 alpha,13 alpha,14 alpha)-isomer | HMDB | | SERB brand OF naloxone hydrochloride | HMDB | | Bristol myers squibb brand OF naloxone hydrochloride | HMDB | | Curamed, naloxon | HMDB | | Lamepro brand OF naloxone hydrochloride | HMDB | | MRZ 2593BR | HMDB | | Nalone | HMDB | | Naloxon-ratiopharm | HMDB | | Hydrobromide, naloxone | HMDB | | Hydrochloride dihydride, naloxone | HMDB | | Hydrochloride, naloxone | HMDB | | MRZ 2593 BR | HMDB | | MRZ 2593-BR | HMDB | | Naloxon curamed | HMDB | | Naloxone abello | HMDB | | Naloxone hydrochloride dihydride | HMDB | | Naloxone, (5 beta,9 alpha,13 alpha,14 alpha)-isomer | HMDB | | Naloxonratiopharm | HMDB | | Naloxone hydrobromide | HMDB | | Narcan | HMDB | | United drug brand OF naloxone hydrochloride | HMDB |
|
|---|
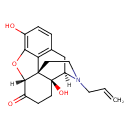
| Chemical Formula | C19H21NO4 |
|---|
| Average Molecular Mass | 327.374 g/mol |
|---|
| Monoisotopic Mass | 327.147 g/mol |
|---|
| CAS Registry Number | 465-65-6 |
|---|
| IUPAC Name | (1S,5R,13R,17S)-10,17-dihydroxy-4-(prop-2-en-1-yl)-12-oxa-4-azapentacyclo[9.6.1.0¹,¹³.0⁵,¹⁷.0⁷,¹⁸]octadeca-7(18),8,10-trien-14-one |
|---|
| Traditional Name | naloxone |
|---|
| SMILES | [H][C@@]12OC3=C(O)C=CC4=C3[C@@]11CCN(CC=C)[C@]([H])(C4)[C@]1(O)CCC2=O |
|---|
| InChI Identifier | InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 |
|---|
| InChI Key | UZHSEJADLWPNLE-GRGSLBFTSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenanthrenes and derivatives. These are polycyclic compounds containing a phenanthrene moiety, which is a tricyclic aromatic compound with three non-linearly fused benzene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenanthrenes and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Phenanthrenes and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenanthrene
- Isoquinolone
- Tetralin
- Coumaran
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Aralkylamine
- Piperidine
- Cyclic alcohol
- Tertiary alcohol
- 1,2-aminoalcohol
- Ketone
- Tertiary aliphatic amine
- Tertiary amine
- Ether
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Carbonyl group
- Organooxygen compound
- Alcohol
- Organic oxygen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a6r-9042000000-3da4b2172456d62748f0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-004i-9703600000-474943cf0224b80b9bab | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-00di-0900000000-6b7de93b51a79132630f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-004i-0139000000-e70921e0765c3389ce85 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0790000000-385321bfef7553f8994a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0009000000-2b2b5b609cf6e1a1d0a7 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0019000000-fdc81a17ea0adfa1d6b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03fr-0009000000-1e89424c27afc0579ffc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dl-5079000000-7ad11d1224dead9d51b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9030000000-102851f487961aeb63cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-8c2763e72d5fce389611 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-0049000000-d9a6f9d54d49fda8a151 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ku-2090000000-fbfe8d648c7bfc3bbe9f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0009000000-354160c39518aa1987e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03fr-0019000000-c4826be6e2657469ccea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00b9-0096000000-16546df9ae1b2c3cee96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-82e8b7e0c70cdc0f9fe6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0009000000-ad63c6111240451f0f01 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00b9-0049000000-d10f137bf09021c8d430 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01183 |
|---|
| HMDB ID | HMDB0015314 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Naloxone |
|---|
| Chemspider ID | 4447644 |
|---|
| ChEBI ID | 7459 |
|---|
| PubChem Compound ID | 5284596 |
|---|
| Kegg Compound ID | C07252 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|