| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:43:57 UTC |
|---|
| Update Date | 2016-11-09 01:15:28 UTC |
|---|
| Accession Number | CHEM016796 |
|---|
| Identification |
|---|
| Common Name | Nalmefene |
|---|
| Class | Small Molecule |
|---|
| Description | Nalmefene is a 6-methylene analogue of naltrexone and opioid system modulator but with no opioid activity . It mediates a partial agonist effect on kappa receptors . It is primarily used in the management of alcohol dependence in adult patients in conjunction with continuous psychosocial support focused on treatment adherence and reducing alcohol consumption when it is exists as the hydrochloride dihydrate form under the trade name Selincro. Nalmefene is orally administered as tablets. Nalmefene works to reduce alcohol consumption in individuals by positive reward effect of alcohol which involves the opioid system, as well as the sedative and dysphoric properties of alcohol .
It is also indicated to prevent or reverse the effects of opioids, including respiratory depression, sedation, and hypotension by acting on the opioid receptor as an antagonist under the trade name Revex for intramuscular, intravenous and subcutaneous injection, where nalmefene hydrochloride is an active ingredient. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
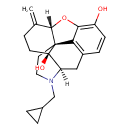
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Selincro | Kegg | | 6-Desoxy-6-methylenenaltrexone | MeSH | | Nalmefene hydrochloride | MeSH | | Revex | MeSH |
|
|---|
| Chemical Formula | C21H25NO3 |
|---|
| Average Molecular Mass | 339.435 g/mol |
|---|
| Monoisotopic Mass | 339.183 g/mol |
|---|
| CAS Registry Number | 55096-26-9 |
|---|
| IUPAC Name | (1S,5R,13S,17S)-4-(cyclopropylmethyl)-14-methylidene-12-oxa-4-azapentacyclo[9.6.1.0¹,¹³.0⁵,¹⁷.0⁷,¹⁸]octadeca-7(18),8,10-triene-10,17-diol |
|---|
| Traditional Name | nalmefene |
|---|
| SMILES | [H][C@@]12OC3=C(O)C=CC4=C3[C@@]11CCN(CC3CC3)[C@]([H])(C4)[C@]1(O)CCC2=C |
|---|
| InChI Identifier | InChI=1S/C21H25NO3/c1-12-6-7-21(24)16-10-14-4-5-15(23)18-17(14)20(21,19(12)25-18)8-9-22(16)11-13-2-3-13/h4-5,13,16,19,23-24H,1-3,6-11H2/t16-,19+,20+,21-/m1/s1 |
|---|
| InChI Key | WJBLNOPPDWQMCH-MBPVOVBZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenanthrenes and derivatives. These are polycyclic compounds containing a phenanthrene moiety, which is a tricyclic aromatic compound with three non-linearly fused benzene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenanthrenes and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Phenanthrenes and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenanthrene
- Tetralin
- Coumaran
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Aralkylamine
- Piperidine
- Cyclic alcohol
- Tertiary alcohol
- 1,2-aminoalcohol
- Tertiary amine
- Tertiary aliphatic amine
- Ether
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Alcohol
- Organic oxygen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fu-2009000000-aca9d9eb1dfc27044aed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-9015000000-337ce45ed49831ae83e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-5458da757dbfc4b9a1cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0019000000-e3e53ca5a418dab59263 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0079-1049000000-05580673a99a6d9c5221 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00l6-4090000000-8087f8c228270eb0a326 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB06230 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Nalmefene |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5284594 |
|---|
| Kegg Compound ID | C08027 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|