| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:43:54 UTC |
|---|
| Update Date | 2016-11-09 01:15:28 UTC |
|---|
| Accession Number | CHEM016794 |
|---|
| Identification |
|---|
| Common Name | Mycophenolate mofetil |
|---|
| Class | Small Molecule |
|---|
| Description | Mycophenolate mofetil, also known as MMF or CellCept, is a prodrug of mycophenolic acid, and classified as a reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH).[A180805] This drug is an immunosuppressant combined with drugs such as [Cyclosporine] and corticosteroids to prevent organ rejection after hepatic, renal, and cardiac transplants.[L7363] It is marketed by Roche Pharmaceuticals and was granted FDA approval for the prophylaxis of transplant rejection in 1995.[A180826] In addition to the above uses, mycophenolate mofetil has also been studied for the treatment of nephritis and other complications of autoimmune diseases. Unlike another immunosuppressant class, the calcineurin inhibitors, MMF generally does not cause nephrotoxicity or fibrosis.[A180799,A180805]
Previously, mycophenolic acid (MPA) was administered to individuals with autoimmune diseases beginning in the 1970s, but was discontinued due to gastrointestinal effects and concerns over carcinogenicity.[A180826] The new semi-synthetic 2-morpholinoethyl ester of MPA was synthesized to avoid the gastrointestinal effects associated with the administration of MPA. It demonstrates an increased bioavailability, a higher efficacy, and reduced gastrointestinal effects when compared to MPA.[A180826] |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
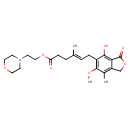
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Morpholinoethyl (e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoate | ChEBI | | Cellcept | ChEBI | | MMF | ChEBI | | Mycophenolic acid morpholinoethyl ester | ChEBI | | RS 61443 | ChEBI | | 2-Morpholinoethyl (e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoic acid | Generator | | Mycophenolate morpholinoethyl ester | Generator | | Mycophenolic acid mofetil | Generator | | Mycophenylate mofetil | HMDB | | Mycophenolate sodium | HMDB | | Myfortic | HMDB | | Sodium mycophenolate | HMDB | | Mycophenolate mofetil hydrochloride | HMDB | | Mofetil hydrochloride, mycophenolate | HMDB | | Mofetil, mycophenolate | HMDB | | Mycophenolate, sodium | HMDB | | Mycophenolic acid | HMDB |
|
|---|
| Chemical Formula | C23H31NO7 |
|---|
| Average Molecular Mass | 433.495 g/mol |
|---|
| Monoisotopic Mass | 433.210 g/mol |
|---|
| CAS Registry Number | 128794-94-5 |
|---|
| IUPAC Name | 2-(morpholin-4-yl)ethyl (4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydro-2-benzofuran-5-yl)-4-methylhex-4-enoate |
|---|
| Traditional Name | mycophenolate mofetil |
|---|
| SMILES | COC1=C(C\C=C(/C)CCC(=O)OCCN2CCOCC2)C(O)=C2C(=O)OCC2=C1C |
|---|
| InChI Identifier | InChI=1S/C23H31NO7/c1-15(5-7-19(25)30-13-10-24-8-11-29-12-9-24)4-6-17-21(26)20-18(14-31-23(20)27)16(2)22(17)28-3/h4,26H,5-14H2,1-3H3/b15-4+ |
|---|
| InChI Key | RTGDFNSFWBGLEC-SYZQJQIISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phthalides. Phthalides are compounds containing a 3-hydrocarbylidene-2-benzofuran-1(3H)-one moiety,. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Isocoumarans |
|---|
| Sub Class | Isobenzofuranones |
|---|
| Direct Parent | Phthalides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phthalide
- Anisole
- Alkyl aryl ether
- Fatty acid ester
- Dicarboxylic acid or derivatives
- Morpholine
- Oxazinane
- Fatty acyl
- Benzenoid
- Vinylogous acid
- Amino acid or derivatives
- Carboxylic acid ester
- Lactone
- Tertiary amine
- Tertiary aliphatic amine
- Ether
- Dialkyl ether
- Carboxylic acid derivative
- Oxacycle
- Azacycle
- Organonitrogen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Organic oxide
- Organopnictogen compound
- Amine
- Carbonyl group
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0w4i-2393200000-d549469b4d276b82f0a4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0itd-4629600000-670072fd9bb0a49d7de1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0019-1741900000-022d7009668cda105e50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0743900000-133a311844263f919bdd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-1961100000-5da439d2a9f8cae8f445 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03xr-9740100000-6af6a8b42b2977762a4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f89-0419700000-cbea334d70b0f1f93d90 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ue9-2859500000-742b323b12fbfbe7a502 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fe3-9564000000-dd2d4e0510f30a121c4e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0020900000-038b179f46cd4955dd10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0291200000-8369d80f2ca22af88fba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0960100000-828ad4ec3f0767246892 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0501900000-6ec023ec52bc4a8e5ea1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004l-2921100000-7173e63d30a5885f9a87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00or-1590000000-77b54540de37ccf344ec | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00688 |
|---|
| HMDB ID | HMDB0014826 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Mycophenolate_mofetil |
|---|
| Chemspider ID | 4444535 |
|---|
| ChEBI ID | 8764 |
|---|
| PubChem Compound ID | 5281078 |
|---|
| Kegg Compound ID | C07908 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Picard N, Cresteil T, Premaud A, Marquet P: Characterization of a phase 1 metabolite of mycophenolic acid produced by CYP3A4/5. Ther Drug Monit. 2004 Dec;26(6):600-8. | | 2. Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, Taylor RS: Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study. Health Technol Assess. 2005 May;9(21):1-179, iii-iv. | | 3. FDA label | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=11099793 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=11490743 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=15572389 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=15992049 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=16979992 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=19858585 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=21180633 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=21710356 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=22081165 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=22294686 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=22310598 | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=22417996 | | 16. https://www.ncbi.nlm.nih.gov/pubmed/?term=22460418 | | 17. https://www.ncbi.nlm.nih.gov/pubmed/?term=22560143 | | 18. https://www.ncbi.nlm.nih.gov/pubmed/?term=8826401 | | 19. https://www.ncbi.nlm.nih.gov/pubmed/?term=9274835 |
|
|---|