| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:43:42 UTC |
|---|
| Update Date | 2016-11-09 01:15:28 UTC |
|---|
| Accession Number | CHEM016788 |
|---|
| Identification |
|---|
| Common Name | Metyrosine |
|---|
| Class | Small Molecule |
|---|
| Description | An L-tyrosine derivative that consists of L-tyrosine bearing an additional methyl substituent at position 2. An inhibitor of the enzyme tyrosine 3-monooxygenase, and consequently of the synthesis of catecholamines. It is used to control the symptoms of excessive sympathetic stimulation in patients with pheochromocytoma. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
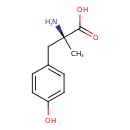
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-alpha-Methyl-L-tyrosine | ChEBI | | (S)-alpha-Methyltyrosine | ChEBI | | alpha-Methyl-L-p-tyrosine | ChEBI | | alpha-Methyl-p-tyrosine | ChEBI | | alpha-Methyl-para-tyrosine | ChEBI | | alpha-Methyltyrosine | ChEBI | | L-alpha-Methyltyrosine | ChEBI | | Methyltyrosine | ChEBI | | Metirosina | ChEBI | | Metirosine | ChEBI | | Metirosinum | ChEBI | | Demser | Kegg | | (-)-a-Methyl-L-tyrosine | Generator | | (-)-Α-methyl-L-tyrosine | Generator | | (S)-a-Methyltyrosine | Generator | | (S)-Α-methyltyrosine | Generator | | a-Methyl-L-p-tyrosine | Generator | | Α-methyl-L-p-tyrosine | Generator | | a-Methyl-p-tyrosine | Generator | | Α-methyl-p-tyrosine | Generator | | a-Methyl-para-tyrosine | Generator | | Α-methyl-para-tyrosine | Generator | | a-Methyltyrosine | Generator | | Α-methyltyrosine | Generator | | L-a-Methyltyrosine | Generator | | L-Α-methyltyrosine | Generator | | alpha Methyltyrosine hydrochloride | HMDB | | alpha-Methyltyrosine, (+,-)-isomer | HMDB | | alpha MPT | HMDB | | alpha Methyl para tyrosine | HMDB | | alpha-MPT | HMDB | | alpha-Methyltyrosine, (D,L)-isomer | HMDB | | alpha-Methyltyrosine, (L)-isomer | HMDB | | Hydrochloride, alpha-methyltyrosine | HMDB | | Metyrosine merck brand | HMDB | | Racemetirosine | HMDB | | alpha Methyl p tyrosine | HMDB | | Merck brand OF metyrosine | HMDB | | Merck sharp and dohme brand OF metyrosine | HMDB | | alpha Methyltyrosine | HMDB | | alpha-Methyltyrosine hydrochloride | HMDB | | alpha-Methyl- DL-tyrosine | HMDB | | a-Methyl-L-tyrosine | HMDB | | Α-methyl-L-tyrosine | HMDB | | Metyrosine | ChEBI |
|
|---|
| Chemical Formula | C10H13NO3 |
|---|
| Average Molecular Mass | 195.215 g/mol |
|---|
| Monoisotopic Mass | 195.090 g/mol |
|---|
| CAS Registry Number | 672-87-7 |
|---|
| IUPAC Name | (2S)-2-amino-3-(4-hydroxyphenyl)-2-methylpropanoic acid |
|---|
| Traditional Name | metyrosine |
|---|
| SMILES | C[C@](N)(CC1=CC=C(O)C=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H13NO3/c1-10(11,9(13)14)6-7-2-4-8(12)5-3-7/h2-5,12H,6,11H2,1H3,(H,13,14)/t10-/m0/s1 |
|---|
| InChI Key | NHTGHBARYWONDQ-JTQLQIEISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpropanoic acids. Phenylpropanoic acids are compounds with a structure containing a benzene ring conjugated to a propanoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Phenylpropanoic acids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Phenylpropanoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-phenylpropanoic-acid
- Alpha-amino acid
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- D-alpha-amino acid
- Phenylpropane
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Amino acid or derivatives
- Amino acid
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Organonitrogen compound
- Primary aliphatic amine
- Organooxygen compound
- Primary amine
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052f-4900000000-946ec0a9cb27d9dde874 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00fu-9651000000-11b1bed7129ec3e5c27a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-7b2e7907b466193628f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0900000000-8ee69272ff1683e7b679 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6r-3900000000-95f4506f225bff0a13e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-3900000000-9aec0e31d6c52c6d4218 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9500000000-ea5a5dcd5c96018a361e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kac-7900000000-25650809bb77fee5eb83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ug1-0900000000-01322d7fad379ffae336 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-053r-0900000000-0f9c6bd0ffbe22e32c94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9200000000-30cb5b9f46a657686267 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6x-1900000000-3caf5c634bee6695a462 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-3900000000-a9dda666c5606b501480 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0536-7900000000-2d7f96942196ae0b76e0 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00765 |
|---|
| HMDB ID | HMDB0014903 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Metirosine |
|---|
| Chemspider ID | 390103 |
|---|
| ChEBI ID | 6912 |
|---|
| PubChem Compound ID | 441350 |
|---|
| Kegg Compound ID | C07921 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|