| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:43:21 UTC |
|---|
| Update Date | 2016-11-09 01:15:28 UTC |
|---|
| Accession Number | CHEM016773 |
|---|
| Identification |
|---|
| Common Name | Methandrostenolone |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
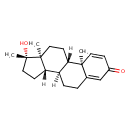
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methandrostenolone | Kegg | | Dianabol | Kegg | | 1, 2-Dehydro-17-methyltestosterone | HMDB | | 1,2-Dehydro-17-methyltestosterone | HMDB | | 1-Dehydro-17-alpha-methyltestosterone | HMDB | | 1-Dehydro-17-methyl-testosterone | HMDB | | 1-Dehydro-17-methyltestosterone | HMDB | | 1-Dehydro-17alpha-methyltestosterone | HMDB | | 1-Dehydromethyltestosterone | HMDB | | 17-alpha-Methyl-1-dehydrotestosterone | HMDB | | 17-alpha-Methylandrostra-1,4-dien-3-one | HMDB | | 17-beta-Hydroxy-17-alpha-methylandrostra-1,4-dien-3-one | HMDB | | 17-beta-Hydroxy-17-methyl-androsta-1,4-dien-3-one | HMDB | | 17-Hydroxy-17-methyl-(17beta)-androsta-1,4-dien-3-one | HMDB | | 17-Hydroxy-17-methyl-(17beta)-androsta-1,4-diene-3-one | HMDB | | 17-Hydroxy-17-methylandrosta-1,4-dien-3-one | HMDB | | 17-Hydroxy-17-methylandrosta-1,4-dien-3-one (acd/name 4.0) | HMDB | | 17alpha-Methyl-1-dehydrotestosterone | HMDB | | 17alpha-Methyl-androsta-1,4-dien-3-one | HMDB | | 17beta-Hydroxy-17-methyl-androsta-1,4-dien-3-one | HMDB | | 17beta-Hydroxy-17alpha-methyl-androsta-1,4-dien-3-one | HMDB | | 17beta-Hydroxy-17alpha-methylandrosta-1,4-dien-3-one | HMDB | | Abirol | HMDB | | alpha-Methyltestosterone | HMDB | | Anabolicum medivet | HMDB | | Anabolin | HMDB | | Andoredan | HMDB | | Androsta-1,4-dien-3-one, 17alpha-methyltestosterone | HMDB | | Crein | HMDB | | Danabol | HMDB | | Dehydromethyltestosterone | HMDB | | Dianabole | HMDB | | Encephan | HMDB | | Geabol | HMDB | | Laquo deltaraquo '-17-methyltestosterone | HMDB | | Laquo deltaraquo 1-17alpha-methyltestosterone | HMDB | | MA | HMDB | | Metanabol | HMDB | | Metandienon | HMDB | | Metandienonum | HMDB | | Metandrostenolon | HMDB | | Metandrostenolone | HMDB | | Metastenol | HMDB | | Methandienone | HMDB | | Methandrolone | HMDB | | Methylandrostenolone | HMDB | | Nabolin | HMDB | | Naposim | HMDB | | Nerobol | HMDB | | Nerobolettes | HMDB | | Protobolin | HMDB | | Stenolon | HMDB | | Stenolone | HMDB | | Sterolon | HMDB | | Metandienone | MeSH |
|
|---|
| Chemical Formula | C20H28O2 |
|---|
| Average Molecular Mass | 300.435 g/mol |
|---|
| Monoisotopic Mass | 300.209 g/mol |
|---|
| CAS Registry Number | 72-63-9 |
|---|
| IUPAC Name | (1S,2R,10R,11S,14S,15S)-14-hydroxy-2,14,15-trimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-3,6-dien-5-one |
|---|
| Traditional Name | methandrostenolone |
|---|
| SMILES | [H][C@@]12CC[C@](C)(O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |
|---|
| InChI Identifier | InChI=1S/C20H28O2/c1-18-9-6-14(21)12-13(18)4-5-15-16(18)7-10-19(2)17(15)8-11-20(19,3)22/h6,9,12,15-17,22H,4-5,7-8,10-11H2,1-3H3/t15-,16+,17+,18+,19+,20+/m1/s1 |
|---|
| InChI Key | XWALNWXLMVGSFR-HLXURNFRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
| Direct Parent | Androgens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Androgen-skeleton
- 3-oxo-delta-1,4-steroid
- 3-oxosteroid
- Hydroxysteroid
- Oxosteroid
- 17-hydroxysteroid
- Delta-1,4-steroid
- Cyclic alcohol
- Tertiary alcohol
- Ketone
- Cyclic ketone
- Organooxygen compound
- Alcohol
- Carbonyl group
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dr-0390000000-1e414c4eabc29414d0e6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0a4i-3449000000-7d9eddf9dd32034a3661 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-0195000000-cd99c3926f1c51806265 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f89-0391000000-4feb078ac411e83b962b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0673-2490000000-fe4e2745917a5b052764 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-4dd9ad61e9950fd00b2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-05774bb7634ca3904bde | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0190000000-76ce9f51fc27e05db90b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-344a11f406f20d02412c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-b206ef592909d7276be2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ls-0290000000-ab9017457af2de1bbada | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ue9-0098000000-116963ecf510cf221cb8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0941000000-ad6c00b7f5135373782d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002f-5920000000-d221c969800b1d74f658 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB13586 |
|---|
| HMDB ID | HMDB0041925 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Metandienone |
|---|
| Chemspider ID | 6061 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6429875 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|