| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:42:45 UTC |
|---|
| Update Date | 2016-11-09 01:15:27 UTC |
|---|
| Accession Number | CHEM016755 |
|---|
| Identification |
|---|
| Common Name | Levallorphan |
|---|
| Class | Small Molecule |
|---|
| Description | An opioid antagonist with properties similar to those of naloxone; in addition it also possesses some agonist properties. It should be used cautiously; levallorphan reverses severe opioid-induced respiratory depression but may exacerbate respiratory depression such as that induced by alcohol or other non-opioid central depressants. (From Martindale, The Extra Pharmacopoeia, 30th ed, p683) |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
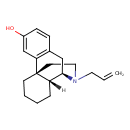
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Lorfan | HMDB | | Naloxiphan | HMDB |
|
|---|
| Chemical Formula | C19H25NO |
|---|
| Average Molecular Mass | 283.408 g/mol |
|---|
| Monoisotopic Mass | 283.194 g/mol |
|---|
| CAS Registry Number | 152-02-3 |
|---|
| IUPAC Name | (1R,9R,10R)-17-(prop-2-en-1-yl)-17-azatetracyclo[7.5.3.0¹,¹⁰.0²,⁷]heptadeca-2(7),3,5-trien-4-ol |
|---|
| Traditional Name | levallorphan |
|---|
| SMILES | [H][C@@]12CCCC[C@@]11CCN(CC=C)[C@@H]2CC2=C1C=C(O)C=C2 |
|---|
| InChI Identifier | InChI=1S/C19H25NO/c1-2-10-20-11-9-19-8-4-3-5-16(19)18(20)12-14-6-7-15(21)13-17(14)19/h2,6-7,13,16,18,21H,1,3-5,8-12H2/t16-,18+,19+/m0/s1 |
|---|
| InChI Key | OZYUPQUCAUTOBP-QXAKKESOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as morphinans. These are polycyclic compounds with a four-ring skeleton with three condensed six-member rings forming a partially hydrogenated phenanthrene moiety, one of which is aromatic while the two others are alicyclic. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Morphinans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Morphinans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Morphinan
- Phenanthrene
- Benzazocine
- Tetralin
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Piperidine
- Benzenoid
- Tertiary aliphatic amine
- Tertiary amine
- Organoheterocyclic compound
- Azacycle
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052f-2090000000-e1bfcb97bfa878802044 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-01tc-3049000000-4352cd28b7455258b001 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-c886e11a52629d3b6ace | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000x-5090000000-a50bd8ddd987c3505bed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9020000000-78f892a0fe089e1dc675 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-d24e2a1bbf1c331446e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001l-0090000000-f90e6f45cb42029ac3f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-1290000000-a9303de3f0f8a329e2f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-de4c3229168dbf071089 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001l-0090000000-6bded21078e814eff199 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0290000000-d27c8821f73ae54df17f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-3c4b9ea0fce2ba47ecaf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0090000000-5b62cdc6cd2bda1045b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ow-1960000000-890603e347ff7eb26f47 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00504 |
|---|
| HMDB ID | HMDB0014647 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Levallorphan |
|---|
| Chemspider ID | 10481920 |
|---|
| ChEBI ID | 804148 |
|---|
| PubChem Compound ID | 5359371 |
|---|
| Kegg Compound ID | C07069 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|