| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:41:56 UTC |
|---|
| Update Date | 2016-11-09 01:15:27 UTC |
|---|
| Accession Number | CHEM016734 |
|---|
| Identification |
|---|
| Common Name | Iodipamide |
|---|
| Class | Small Molecule |
|---|
| Description | An organoiodine compound that is 3-amino-2,4,6-triiodobenzoic acid in which one of the amino hydrogens is substituted by a 6-(3-carboxy-2,4,6-triiodoanilino)-6-oxohexanoyl group. It is a water-soluble radiographic contrast media for cholecystography and intravenous cholangiography. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
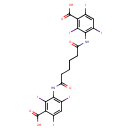
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,3'-(Adipoyldiimino)bis(2,4,6-triiodobenzoic acid) | ChEBI | | Adipiodon | ChEBI | | Adipiodona | ChEBI | | Adipiodonum | ChEBI | | Bilignost | ChEBI | | Bilignostum | ChEBI | | Biligrafin | ChEBI | | Biligrafine | ChEBI | | Bilipolinum | ChEBI | | Cholografin | ChEBI | | Cholospect | ChEBI | | Endocistobil | ChEBI | | Endographin | ChEBI | | Transbilix | ChEBI | | Adipiodone | Kegg | | 3,3'-(Adipoyldiimino)bis(2,4,6-triiodobenzoate) | Generator | | 3,3'-[(1,6-Dioxohexane-1,6-diyl)diimino]bis(2,4,6-triiodobenzoic acid) | HMDB | | IDB | HMDB | | Iodipamic acid | HMDB |
|
|---|
| Chemical Formula | C20H14I6N2O6 |
|---|
| Average Molecular Mass | 1139.762 g/mol |
|---|
| Monoisotopic Mass | 1139.512 g/mol |
|---|
| CAS Registry Number | 606-17-7 |
|---|
| IUPAC Name | 3-{5-[(3-carboxy-2,4,6-triiodophenyl)carbamoyl]pentanamido}-2,4,6-triiodobenzoic acid |
|---|
| Traditional Name | iodipamide |
|---|
| SMILES | OC(=O)C1=C(I)C(NC(=O)CCCCC(=O)NC2=C(I)C=C(I)C(C(O)=O)=C2I)=C(I)C=C1I |
|---|
| InChI Identifier | InChI=1S/C20H14I6N2O6/c21-7-5-9(23)17(15(25)13(7)19(31)32)27-11(29)3-1-2-4-12(30)28-18-10(24)6-8(22)14(16(18)26)20(33)34/h5-6H,1-4H2,(H,27,29)(H,28,30)(H,31,32)(H,33,34) |
|---|
| InChI Key | FFINMCNLQNTKLU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acylaminobenzoic acid and derivatives. These are derivatives of amino benzoic acid derivatives where the amine group is N-acylated. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Acylaminobenzoic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acylaminobenzoic acid or derivatives
- 2-halobenzoic acid or derivatives
- 4-halobenzoic acid or derivatives
- Halobenzoic acid or derivatives
- 2-halobenzoic acid
- 4-halobenzoic acid
- Halobenzoic acid
- Benzoic acid
- Anilide
- 1-carboxy-2-haloaromatic compound
- Benzoyl
- N-arylamide
- Halobenzene
- Iodobenzene
- Dicarboxylic acid or derivatives
- Aryl iodide
- Aryl halide
- Fatty acyl
- Fatty amide
- Vinylogous halide
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid
- Carboxylic acid derivative
- Organonitrogen compound
- Organooxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organohalogen compound
- Organoiodide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-2900020000-b5a5aa037fa7d817df43 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00tg-1800093000-021cd14ecd426c78058a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfs-6000190000-ab797bf6191c4bfd4c3f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000l-9700010000-f76a1115bde515a53e50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01p9-3900082000-899be96e35a30d50367a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-1000190000-4b000454347cb3aa66af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-d4fd3411cd3835817df6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0900000000-d75e6488bde838e8c58e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066s-2000491102-1f7d63b3993a839ad99f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-bf652d3be877b9723043 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00mx-9400000002-b23b5cd3f71e6c2ab474 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0900110001-f542eee28a08a4f777ee | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB04711 |
|---|
| HMDB ID | HMDB0015581 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | IDB |
|---|
| Wikipedia Link | Adipiodone |
|---|
| Chemspider ID | 3608 |
|---|
| ChEBI ID | 31176 |
|---|
| PubChem Compound ID | 3739 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|