| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:40:48 UTC |
|---|
| Update Date | 2016-11-09 01:15:27 UTC |
|---|
| Accession Number | CHEM016699 |
|---|
| Identification |
|---|
| Common Name | Etidocaine |
|---|
| Class | Small Molecule |
|---|
| Description | An amino acid amide in which 2-butanoic acid and 2,6-dimethylaniline have combined to form the amide bond. Used as a local anaesthetic (amide caine), it has rapid onset and long action properties, similar to bupivacaine, and is given by injection during surgical procedures and during labour and delivery. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
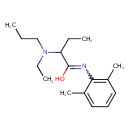
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+-)-2-(Ethylpropylamino)-2',6'-butyroxylidide | ChEBI | | (+-)-N-(2,6-Dimethylphenyl)-2-(ethylpropylamino)butanamide | ChEBI | | Etidocaina | ChEBI | | Etidocainum | ChEBI | | Duranest | MeSH | | 2-(ethylpropylamino)-2',6'-Butyroxylidide | MeSH |
|
|---|
| Chemical Formula | C17H28N2O |
|---|
| Average Molecular Mass | 276.417 g/mol |
|---|
| Monoisotopic Mass | 276.220 g/mol |
|---|
| CAS Registry Number | 36637-18-0 |
|---|
| IUPAC Name | N-(2,6-dimethylphenyl)-2-[ethyl(propyl)amino]butanimidic acid |
|---|
| Traditional Name | etidocaine product |
|---|
| SMILES | CCCN(CC)C(CC)C(O)=NC1=C(C)C=CC=C1C |
|---|
| InChI Identifier | InChI=1S/C17H28N2O/c1-6-12-19(8-3)15(7-2)17(20)18-16-13(4)10-9-11-14(16)5/h9-11,15H,6-8,12H2,1-5H3,(H,18,20) |
|---|
| InChI Key | VTUSIVBDOCDNHS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alpha amino acid amides. These are amide derivatives of alpha amino acids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acid amides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid amide
- Anilide
- N-arylamide
- M-xylene
- Xylene
- Monocyclic benzene moiety
- Fatty amide
- Benzenoid
- Fatty acyl
- Carboxamide group
- Secondary carboxylic acid amide
- Tertiary amine
- Tertiary aliphatic amine
- Amine
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic nitrogen compound
- Carbonyl group
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fmj-3910000000-db88968f7e8b8521d31a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00b9-1970000000-c8e2000362b4a51d8261 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00fr-1900000000-c11c0eca2c66f7aa9c69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9300000000-3baddd74621733d93461 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0290000000-e315f2652a3b1978535d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05dr-1950000000-7de019524f03a938804b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00xr-4900000000-15702a2a8dffb90723f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0960000000-a33b9f17585a221289f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ufr-1900000000-f049bccb62b254931d28 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gvo-6900000000-81ad97f4b02324678b63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-5d9d72791f8fcf054eaa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00or-1930000000-46124d3bcd87b2bb4ec5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-4900000000-db07b84ccbeb36002565 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB08987 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Etidocaine |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 4904 |
|---|
| PubChem Compound ID | 37497 |
|---|
| Kegg Compound ID | C07530 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|