| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:39:58 UTC |
|---|
| Update Date | 2016-11-09 01:15:27 UTC |
|---|
| Accession Number | CHEM016670 |
|---|
| Identification |
|---|
| Common Name | Dimetacrine |
|---|
| Class | Small Molecule |
|---|
| Description | Dimetacrine is also known as dimethacrine or acripamine. It is marketed under the names Istonil, Istonyl, Linostil, and Miroistonil. Dimetacrine is a tricyclic antidepressant (TCA) with imipramine-like effects used in Europe for the treatment of depression. Dimetacrine is no longer used in Japan. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
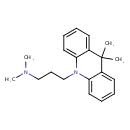
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 10-(3-(Dimethylamino)propyl)-9,9-dimethylacridan tartrate | MeSH | | 3-(9,9-Dimethyl-10(9H)-acridinyl)-N,N-dimethyl-1-propanamine | MeSH | | Dimetacrine bitartate | MeSH | | Dimetacrine tartate | MeSH | | Dimethacrine | MeSH | | Dimethacrine tartrate | MeSH | | Dimethacrine tartrate, (1:1)(R-(r*,r*))-isomer | MeSH | | Istonil | MeSH | | Miroistonil | MeSH | | Isotonil | MeSH |
|
|---|
| Chemical Formula | C20H26N2 |
|---|
| Average Molecular Mass | 294.434 g/mol |
|---|
| Monoisotopic Mass | 294.210 g/mol |
|---|
| CAS Registry Number | 4757-55-5 |
|---|
| IUPAC Name | [3-(9,9-dimethyl-9,10-dihydroacridin-10-yl)propyl]dimethylamine |
|---|
| Traditional Name | dimetacrine |
|---|
| SMILES | CN(C)CCCN1C2=CC=CC=C2C(C)(C)C2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C20H26N2/c1-20(2)16-10-5-7-12-18(16)22(15-9-14-21(3)4)19-13-8-6-11-17(19)20/h5-8,10-13H,9,14-15H2,1-4H3 |
|---|
| InChI Key | RYQOGSFEJBUZBX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acridines. These are organic compounds containing the acridine moiety, a linear tricyclic heterocycle which consists of two benzene rings joined by a pyridine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Benzoquinolines |
|---|
| Direct Parent | Acridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acridine
- Alkyldiarylamine
- Tertiary aliphatic/aromatic amine
- Benzenoid
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9580000000-a0b4b0a04fe5d685c54d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-1090000000-f1ec989b044f9c24d5bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f72-5090000000-1633096ac4395c74338e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000f-9130000000-eb4f001b2bd55e81c50a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-d1f3a00dc93e3e42dbd5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052f-0090000000-e69ae99ca00798b20846 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aor-2790000000-99e516e9016bfd8f01f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-19fde7b235562f81ace5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-1090000000-72b162b3d9dc56876d50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9360000000-6b017f602090f565ab8f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-d7e41f50346ef7ead2a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0090000000-1b4ca5fb6e195df60ca3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-0790000000-3ea1bf32911269e8d62b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB08996 |
|---|
| HMDB ID | HMDB0251373 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Dimetacrine |
|---|
| Chemspider ID | 85085 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 94280 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|