| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:39:46 UTC |
|---|
| Update Date | 2016-11-09 01:15:26 UTC |
|---|
| Accession Number | CHEM016663 |
|---|
| Identification |
|---|
| Common Name | Diatrizoate sodium |
|---|
| Class | Small Molecule |
|---|
| Description | The sodium salt of a benzoic acid having iodo substituents at the 2-, 4- and 6-positions and acetamido substituents at the 3- and 5-positions. It is used, often as a mixture with the meglumine salt, as an X-ray contrast medium in gastrointestinal studies, angiography, and urography. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,5-Diacetylamino-2,4,6-trijodbenzosaeure natrium | ChEBI | | Amidotrizoate | ChEBI | | Amidotrizoate de sodium | ChEBI | | Amidotrizoato sodico | ChEBI | | Conray 35 | ChEBI | | Diatriazoate | ChEBI | | Diatrizoate sodium | ChEBI | | Diatrizoate sodium salt | ChEBI | | Diatrizoic acid sodium salt | ChEBI | | Histopaque | ChEBI | | Hpaque - cysto | ChEBI | | Hypaque | ChEBI | | Hypaque cysto | ChEBI | | Hypaque sodium | ChEBI | | MD 50 | ChEBI | | MD-50 | ChEBI | | Monosodium 3,5-diacetamido-2,4,6-triiodobenzoate | ChEBI | | Natrii amidotrizoas | ChEBI | | Sodium diacetyldiaminetriiodobenzoate | ChEBI | | Sodium diatrizoate | ChEBI | | Triombrin | ChEBI | | Triombrine | ChEBI | | Urovist sodium | ChEBI | | Vascoray | ChEBI | | Amidotrizoic acid | Generator | | Amidotrizoic acid de sodium | Generator | | Diatriazoic acid | Generator | | Diatrizoic acid sodium | Generator | | Monosodium 3,5-diacetamido-2,4,6-triiodobenzoic acid | Generator | | Sodium diacetyldiaminetriiodobenzoic acid | Generator | | Sodium diatrizoic acid | Generator | | Sodium amidotrizoic acid | Generator |
|
|---|
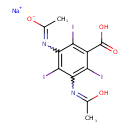
| Chemical Formula | C11H8I3N2NaO4 |
|---|
| Average Molecular Mass | 635.895 g/mol |
|---|
| Monoisotopic Mass | 635.752 g/mol |
|---|
| CAS Registry Number | 737-31-5 |
|---|
| IUPAC Name | sodium N-{3-carboxy-5-[(1-hydroxyethylidene)amino]-2,4,6-triiodophenyl}ethanecarboximidate |
|---|
| Traditional Name | sodium N-{3-carboxy-5-[(1-hydroxyethylidene)amino]-2,4,6-triiodophenyl}ethanecarboximidate |
|---|
| SMILES | [Na+].CC(O)=NC1=C(I)C(C(O)=O)=C(I)C(N=C(C)[O-])=C1I |
|---|
| InChI Identifier | InChI=1S/C11H9I3N2O4.Na/c1-3(17)15-9-6(12)5(11(19)20)7(13)10(8(9)14)16-4(2)18;/h1-2H3,(H,15,17)(H,16,18)(H,19,20);/q;+1/p-1 |
|---|
| InChI Key | ZEYOIOAKZLALAP-UHFFFAOYSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acylaminobenzoic acid and derivatives. These are derivatives of amino benzoic acid derivatives where the amine group is N-acylated. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Acylaminobenzoic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acylaminobenzoic acid or derivatives
- O-haloacetanilide
- P-haloacetanilide
- Haloacetanilide
- Acetanilide
- Halobenzoic acid
- 4-halobenzoic acid
- 2-halobenzoic acid
- Halobenzoic acid or derivatives
- 2-halobenzoic acid or derivatives
- 4-halobenzoic acid or derivatives
- Benzoic acid
- N-acetylarylamine
- Anilide
- N-arylamide
- Benzoyl
- 1-carboxy-2-haloaromatic compound
- Halobenzene
- Iodobenzene
- Aryl halide
- Aryl iodide
- Acetamide
- Vinylogous halide
- Secondary carboxylic acid amide
- Carboxamide group
- Carboxylic acid salt
- Organic alkali metal salt
- Organic metal halide
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic salt
- Organic nitrogen compound
- Organohalogen compound
- Organoiodide
- Organonitrogen compound
- Organooxygen compound
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organic sodium salt
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000b-0000095000-bb356e7c3c923855f43d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0000090000-70f44c0b27137e61df80 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kdi-3000090000-63221b137816da667ed4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001l-0000096000-76acabd7832d83c47ab9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000x-1000097000-e808cea56d1d9eced761 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udl-1000091000-744d4d0649ff45daeb9b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT000267 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Diatrizoate |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 53692 |
|---|
| PubChem Compound ID | 12916 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|