| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:38:35 UTC |
|---|
| Update Date | 2016-11-09 01:15:26 UTC |
|---|
| Accession Number | CHEM016617 |
|---|
| Identification |
|---|
| Common Name | Cefoxitin |
|---|
| Class | Small Molecule |
|---|
| Description | A semisynthetic cephamycin antibiotic which, in addition to the methoxy group at the 7alpha position, has 2-thienylacetamido and carbamoyloxymethyl side-groups. It is resistant to beta-lactamase. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
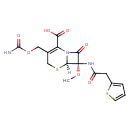
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (6R,7S)-3-[(Carbamoyloxy)methyl]-7-methoxy-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | ChEBI | | Cefoxitina | ChEBI | | Cefoxitine | ChEBI | | Cefoxitinum | ChEBI | | Ceftoxitin | ChEBI | | Cephoxitin | ChEBI | | CFX | ChEBI | | Rephoxitin | ChEBI | | (6R,7S)-3-[(Carbamoyloxy)methyl]-7-methoxy-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate | Generator | | MSD Brand OF cefoxitin sodium | HMDB | | Sodium, cefoxitin | HMDB | | Merck brand OF cefoxitin sodium | HMDB | | Cefoxitin sodium | HMDB | | Mefoxitin | HMDB | | Mefoxin | HMDB | | Merck frosst brand OF cefoxitin sodium | HMDB | | Merck sharp and dohme brand OF cefoxitin sodium | HMDB | | Méfoxin | HMDB |
|
|---|
| Chemical Formula | C16H17N3O7S2 |
|---|
| Average Molecular Mass | 427.452 g/mol |
|---|
| Monoisotopic Mass | 427.051 g/mol |
|---|
| CAS Registry Number | 35607-66-0 |
|---|
| IUPAC Name | (6R,7S)-3-[(carbamoyloxy)methyl]-7-methoxy-8-oxo-7-[2-(thiophen-2-yl)acetamido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
|---|
| Traditional Name | cefoxitin |
|---|
| SMILES | [H][C@]12SCC(COC(N)=O)=C(N1C(=O)[C@]2(NC(=O)CC1=CC=CS1)OC)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C16H17N3O7S2/c1-25-16(18-10(20)5-9-3-2-4-27-9)13(23)19-11(12(21)22)8(6-26-15(17)24)7-28-14(16)19/h2-4,14H,5-7H2,1H3,(H2,17,24)(H,18,20)(H,21,22)/t14-,16+/m1/s1 |
|---|
| InChI Key | WZOZEZRFJCJXNZ-ZBFHGGJFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid esters. Steroid esters are compounds containing a steroid moiety which bears a carboxylic acid ester group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroid esters |
|---|
| Direct Parent | Steroid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroid ester
- Androstane-skeleton
- N-methylpiperazine
- N-alkylpiperazine
- 1,4-diazinane
- Dicarboxylic acid or derivatives
- Piperazine
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Amino acid or derivatives
- Carboxylic acid ester
- Tertiary amine
- Tertiary aliphatic amine
- Azacycle
- Carboxylic acid derivative
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Carbonyl group
- Organic nitrogen compound
- Organic salt
- Organic cation
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0005-9324000000-05cb1df27c44fb10d1f0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-006w-9111500000-bf31bd6d1e9af793d957 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-0152900000-dcfff8fc4fd3f312364a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0096-0920000000-12829e022c644e05c20d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-4930000000-b840384f3eb09ebca7c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-9132100000-8247f1786ca63286b408 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9111000000-3136ee10c61ca55fabbc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9510000000-6fe60420b7da0fc35ee7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-016r-0009800000-0aab03e94594dab686d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0171-1059200000-d98c8e6245c4548a77ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000j-9277000000-b3dc1748967d8be6f876 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fb9-0015900000-11bd6d703d43a001e23e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-016r-2092200000-0fab277122b825145b75 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0005-9220000000-b8f3a5c616f492419695 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01331 |
|---|
| HMDB ID | HMDB0015426 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | CFX |
|---|
| Wikipedia Link | Cefoxitin |
|---|
| Chemspider ID | 389981 |
|---|
| ChEBI ID | 209807 |
|---|
| PubChem Compound ID | 441199 |
|---|
| Kegg Compound ID | C06887 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|