| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:38:22 UTC |
|---|
| Update Date | 2016-11-09 01:15:26 UTC |
|---|
| Accession Number | CHEM016608 |
|---|
| Identification |
|---|
| Common Name | Carminomycin |
|---|
| Class | Small Molecule |
|---|
| Description | A toxic anthracycline antibiotic that is produced by Actinomadura carminata and also has potent antineoplastic activity. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
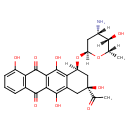
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Carubicin | ChEBI | | CCRIS 961 | ChEBI | | Karminomycin | ChEBI | | O-Demethyldaunomycin | ChEBI | | Carminomicin | MeSH | | Carminomycin II | MeSH | | Hydrochloride, carubicin | MeSH | | Rubeomycin a1 | MeSH | | Carminomycin I | MeSH | | Demethyldaunomycin | MeSH | | Carminomycin III | MeSH | | Demethyldaunorubicin | MeSH | | Rubeomycin a | MeSH | | Carubicin hydrochloride | MeSH | | Karminomicin | MeSH |
|
|---|
| Chemical Formula | C26H27NO10 |
|---|
| Average Molecular Mass | 513.499 g/mol |
|---|
| Monoisotopic Mass | 513.163 g/mol |
|---|
| CAS Registry Number | 50935-04-1 |
|---|
| IUPAC Name | (8S,10S)-8-acetyl-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy}-1,6,8,11-tetrahydroxy-5,7,8,9,10,12-hexahydrotetracene-5,12-dione |
|---|
| Traditional Name | carminomycin |
|---|
| SMILES | [H][C@]1(N)C[C@]([H])(O[C@@]2([H])C[C@@](O)(CC3=C(O)C4=C(C(O)=C23)C(=O)C2=C(C=CC=C2O)C4=O)C(C)=O)O[C@@]([H])(C)[C@@]1([H])O |
|---|
| InChI Identifier | InChI=1S/C26H27NO10/c1-9-21(30)13(27)6-16(36-9)37-15-8-26(35,10(2)28)7-12-18(15)25(34)20-19(23(12)32)22(31)11-4-3-5-14(29)17(11)24(20)33/h3-5,9,13,15-16,21,29-30,32,34-35H,6-8,27H2,1-2H3/t9-,13-,15-,16-,21+,26-/m0/s1 |
|---|
| InChI Key | XREUEWVEMYWFFA-CSKJXFQVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as anthracyclines. These are polyketides containing a tetracenequinone ring structure with a sugar attached by glycosidic linkage. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Anthracyclines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Anthracyclines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Anthracycline
- Anthracyclinone-skeleton
- Aminoglycoside core
- Tetracenequinone
- 9,10-anthraquinone
- 1,4-anthraquinone
- Anthracene
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Tetralin
- Aryl ketone
- Amino saccharide
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Monosaccharide
- Oxane
- Tertiary alcohol
- Alpha-hydroxy ketone
- Vinylogous acid
- Secondary alcohol
- 1,2-aminoalcohol
- Ketone
- Polyol
- Organoheterocyclic compound
- Acetal
- Oxacycle
- Alcohol
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Primary aliphatic amine
- Organic nitrogen compound
- Amine
- Organonitrogen compound
- Primary amine
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03y1-0009640000-34d19b4f5d8c12af8e7f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-0319100000-ba8046944f882fb30aea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-6239000000-14d7102b462bb57eb98a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1106490000-39f06fccf321ebc6d072 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001l-2109400000-2c91fca3d223bba58a7f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-3109000000-1031d4a1fb1f8e3929a9 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-15734 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Carubicin |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 31359 |
|---|
| PubChem Compound ID | 443831 |
|---|
| Kegg Compound ID | C12432 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|