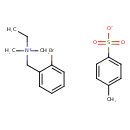| (2-Bromobenzyl)ethyldimethylammonium toluene-4-sulfonate | ChEBI |
| (2-Bromobenzyl)ethyldimethylammonium tosylate | ChEBI |
| (O-Bromobenzyl)ethyldimethylammonium p-toluenesulfonate | ChEBI |
| 2-Bromo-N-ethyl-N,N-dimethylbenzenemethanaminium 4-methylbenzenesulfonate | ChEBI |
| Bretylii tosilas | ChEBI |
| Dimethylethyl-O-bromobenzylammonium-p-toluenesulphonate | ChEBI |
| N-Ethyl-N-O-bromobenzyl-N,N-dimethylammonium tosylate | ChEBI |
| Tosilate de bretylium | ChEBI |
| Tosilato de bretilio | ChEBI |
| Bretylium tosilate | Kegg |
| Bretylol | Kegg |
| (2-Bromobenzyl)ethyldimethylammonium toluene-4-sulfonic acid | Generator |
| (2-Bromobenzyl)ethyldimethylammonium toluene-4-sulphonate | Generator |
| (2-Bromobenzyl)ethyldimethylammonium toluene-4-sulphonic acid | Generator |
| (2-Bromobenzyl)ethyldimethylammonium tosylic acid | Generator |
| (O-Bromobenzyl)ethyldimethylammonium p-toluenesulfonic acid | Generator |
| (O-Bromobenzyl)ethyldimethylammonium p-toluenesulphonate | Generator |
| (O-Bromobenzyl)ethyldimethylammonium p-toluenesulphonic acid | Generator |
| 2-Bromo-N-ethyl-N,N-dimethylbenzenemethanaminium 4-methylbenzenesulfonic acid | Generator |
| 2-Bromo-N-ethyl-N,N-dimethylbenzenemethanaminium 4-methylbenzenesulphonate | Generator |
| 2-Bromo-N-ethyl-N,N-dimethylbenzenemethanaminium 4-methylbenzenesulphonic acid | Generator |
| Dimethylethyl-O-bromobenzylammonium-p-toluenesulfonate | Generator |
| Dimethylethyl-O-bromobenzylammonium-p-toluenesulfonic acid | Generator |
| Dimethylethyl-O-bromobenzylammonium-p-toluenesulphonic acid | Generator |
| N-Ethyl-N-O-bromobenzyl-N,N-dimethylammonium tosylic acid | Generator |
| Tosilic acid de bretylium | Generator |
| Bretylium tosilic acid | Generator |
| Bretylium tosylic acid | Generator |
| Ornid | MeSH |
| Bretylate | MeSH |
| (2-Bromophenyl)methyl-ethyl-dimethylazanium;4-methylbenzenesulfonic acid | Generator |
| (2-Bromophenyl)methyl-ethyl-dimethylazanium;4-methylbenzenesulphonate | Generator |
| (2-Bromophenyl)methyl-ethyl-dimethylazanium;4-methylbenzenesulphonic acid | Generator |
| Du pont brand OF bretylium tosilate | MeSH |
| Bretylium tosylate | MeSH |
| GlaxoSmithKline brand OF bretylium tosilate | MeSH |