| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:37:45 UTC |
|---|
| Update Date | 2016-11-09 01:15:26 UTC |
|---|
| Accession Number | CHEM016585 |
|---|
| Identification |
|---|
| Common Name | Bepridil |
|---|
| Class | Small Molecule |
|---|
| Description | A long-acting, non selective, calcium channel blocker with significant anti-anginal activity. The drug produces significant coronary vasodilation and modest peripheral effects. It has antihypertensive and selective anti-arrhythmia activities and acts as a calmodulin antagonist. It is no longer marketed in the United States, as it has been implicated in causing ventricular arrhythmias (ie. Torsade de pointes). |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Bepadin | ChEBI | | Bepridil, (+)-isomer | HMDB | | Bepridil, alpha-isomer | HMDB | | Monohydrochloride, bepridil | HMDB | | Riom brand OF bepridil hydrochloride | HMDB | | Vascor | HMDB | | 1978 CERM | HMDB | | Bepridil monohydrochloride | HMDB | | Bepridil monohydrochloride, monohydrate | HMDB | | Bepridil monohydrochloride, alpha isomer | HMDB | | Bepridil monohydrochloride, alpha-isomer | HMDB | | Bepridil, (-)-isomer | HMDB | | Bepridil, alpha isomer | HMDB | | Cordium | HMDB | | Wallace brand OF bepridil hydrochloride | HMDB | | 1978-CERM | HMDB | | Bepridil, (+-)-isomer | HMDB | | CERM-1978 | HMDB | | Bedapin | HMDB | | CERM 1978 | HMDB | | Monohydrochloride, alpha-isomer bepridil | HMDB | | Nourypharma brand OF bepridil hydrochloride | HMDB | | Unicordium | HMDB |
|
|---|
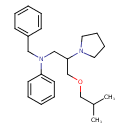
| Chemical Formula | C24H34N2O |
|---|
| Average Molecular Mass | 366.540 g/mol |
|---|
| Monoisotopic Mass | 366.267 g/mol |
|---|
| CAS Registry Number | 64706-54-3 |
|---|
| IUPAC Name | N-benzyl-N-[3-(2-methylpropoxy)-2-(pyrrolidin-1-yl)propyl]aniline |
|---|
| Traditional Name | bepridil |
|---|
| SMILES | CC(C)COCC(CN(CC1=CC=CC=C1)C1=CC=CC=C1)N1CCCC1 |
|---|
| InChI Identifier | InChI=1S/C24H34N2O/c1-21(2)19-27-20-24(25-15-9-10-16-25)18-26(23-13-7-4-8-14-23)17-22-11-5-3-6-12-22/h3-8,11-14,21,24H,9-10,15-20H2,1-2H3 |
|---|
| InChI Key | UIEATEWHFDRYRU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylbenzamines. These are aromatic compounds consisting of a benzyl group that is N-linked to a benzamine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylmethylamines |
|---|
| Direct Parent | Phenylbenzamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylbenzamine
- Benzylamine
- Tertiary aliphatic/aromatic amine
- Aniline or substituted anilines
- Dialkylarylamine
- Aralkylamine
- N-alkylpyrrolidine
- Pyrrolidine
- Tertiary amine
- Tertiary aliphatic amine
- Dialkyl ether
- Ether
- Azacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004m-8690000000-768d16d0b40d5b2932e7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-066u-9327000000-af93fdc247abd705238e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9320000000-2b4cc0379d6875c5c1ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-675ce98f6b729bc83dba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-2019000000-ad43d391dc0021d16521 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05x0-8895000000-f6081c9498fdf024c130 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9220000000-a3433671009f66e28106 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-1309000000-d08b70c49dbd9bec8ecc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014l-9527000000-d456621f5de3ad386cb9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9400000000-94c75315da72259763c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0219000000-f342a2f667dda5fca827 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-07vi-3629000000-ebc015e4f6668c377562 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003r-9822000000-709fc32123a04540e60f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01244 |
|---|
| HMDB ID | HMDB0015374 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Bepridil |
|---|
| Chemspider ID | 2261 |
|---|
| ChEBI ID | 3061 |
|---|
| PubChem Compound ID | 2351 |
|---|
| Kegg Compound ID | C06847 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|