| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:37:43 UTC |
|---|
| Update Date | 2016-11-09 01:15:26 UTC |
|---|
| Accession Number | CHEM016583 |
|---|
| Identification |
|---|
| Common Name | Benzonatate |
|---|
| Class | Small Molecule |
|---|
| Description | The ester obtained by formal condensation of 4-butylaminobenzoic acid with nonaethylene glycol monomethyl ether. Structurally related to procaine and benzocaine, it has an anaesthetic effect on the stretch sensors in the lungs, and is used as a non-narcotic cough suppressant. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
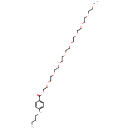
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,5,8,11,14,17,20,23,26-Nonaoxaoctacosan-28-yl p-(butylamino)benzoate | ChEBI | | 2-[2-[2-[2-[2-[2-[2-[2-(2-Methoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethyl 4-butylaminobenzoate | ChEBI | | 3,6,9,12,15,18,21,24,27-Nonaoxaoctacosyl 4-butylaminobenzoate | ChEBI | | 4-(Butylamino)benzoic acid 3,6,9,12,15,18,21,24,27-nonaoxaoctacos-1-yl ester | ChEBI | | Benzonatato | ChEBI | | Benzonatatum | ChEBI | | Benzononatine | ChEBI | | Nonaethyleneglycol monomethyl ether p-N-butylaminobenzoate | ChEBI | | p-Butylaminobenzoic acid omega-O-methylnonaethyleneglycol ester | ChEBI | | Tessalon perles | Kegg | | 2,5,8,11,14,17,20,23,26-Nonaoxaoctacosan-28-yl p-(butylamino)benzoic acid | Generator | | 2-[2-[2-[2-[2-[2-[2-[2-(2-Methoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethyl 4-butylaminobenzoic acid | Generator | | 3,6,9,12,15,18,21,24,27-Nonaoxaoctacosyl 4-butylaminobenzoic acid | Generator | | 4-(Butylamino)benzoate 3,6,9,12,15,18,21,24,27-nonaoxaoctacos-1-yl ester | Generator | | Nonaethyleneglycol monomethyl ether p-N-butylaminobenzoic acid | Generator | | p-Butylaminobenzoate omega-O-methylnonaethyleneglycol ester | Generator | | Benzonatic acid | Generator | | Tessalon | HMDB |
|
|---|
| Chemical Formula | C30H53NO11 |
|---|
| Average Molecular Mass | 603.742 g/mol |
|---|
| Monoisotopic Mass | 603.362 g/mol |
|---|
| CAS Registry Number | 104-31-4 |
|---|
| IUPAC Name | 2,5,8,11,14,17,20,23,26-nonaoxaoctacosan-28-yl 4-(butylamino)benzoate |
|---|
| Traditional Name | benzonatate |
|---|
| SMILES | CCCCNC1=CC=C(C=C1)C(=O)OCCOCCOCCOCCOCCOCCOCCOCCOCCOC |
|---|
| InChI Identifier | InChI=1S/C30H53NO11/c1-3-4-9-31-29-7-5-28(6-8-29)30(32)42-27-26-41-25-24-40-23-22-39-21-20-38-19-18-37-17-16-36-15-14-35-13-12-34-11-10-33-2/h5-8,31H,3-4,9-27H2,1-2H3 |
|---|
| InChI Key | MAFMQEKGGFWBAB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzoic acid esters. These are ester derivatives of benzoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Benzoic acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminobenzoic acid or derivatives
- Benzoate ester
- Benzoyl
- Aniline or substituted anilines
- Phenylalkylamine
- Secondary aliphatic/aromatic amine
- Amino acid or derivatives
- Carboxylic acid ester
- Carboxylic acid derivative
- Secondary amine
- Dialkyl ether
- Monocarboxylic acid or derivatives
- Ether
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Organic nitrogen compound
- Amine
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00ba-4789430000-3e3f5cdbfca7adf27324 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0nmm-2794000000-e2d12296cd52bc5b6c1b | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-004i-2900000000-3718c62c83080a92ccdd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0w4i-3922626000-5dc7fe4297607d083b74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9744340000-9f08af439f9cd3514671 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9665010000-1bf3fbd87ab1a87e011c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6x-0922113000-2d47eaeef80482270092 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0597-2921111000-2cc95d4ee244c9054738 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-4920000000-1c2d9b1acf4ebda28438 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-9321011000-f4806fb7494d3b14849c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9200010000-4fd975b439aea833149f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9600000000-ebbc2bf3aeb5569f4924 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a6r-9731100000-3824f37521f4fc6505b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0btc-9411100000-2057400c582684721e81 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000l-4910000000-a4a3ebc1f733a1ccfe9d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00868 |
|---|
| HMDB ID | HMDB0015006 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Benzonatate |
|---|
| Chemspider ID | 7413 |
|---|
| ChEBI ID | 3032 |
|---|
| PubChem Compound ID | 7699 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|