| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:37:38 UTC |
|---|
| Update Date | 2016-11-09 01:15:26 UTC |
|---|
| Accession Number | CHEM016580 |
|---|
| Identification |
|---|
| Common Name | Bendroflumethiazide |
|---|
| Class | Small Molecule |
|---|
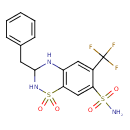
| Description | A sulfonamide consisting of 7-sulfamoyl-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide in which the hydrogen at position 6 is substituted by a trifluoromethyl group and that at position 3 is substituted by a benzyl group. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| +--3-Benzyl-3,4-dihydro-6-(trifluoromethyl)-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide | ChEBI | | 6-Trifluoromethyl-3-benzyl-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide | ChEBI | | Bendrofluazide | ChEBI | | Bendroflumethiazidum | ChEBI | | Bendroflumetiazida | ChEBI | | Benzhydroflumethiazide | ChEBI | | Naturetin | Kegg | | +--3-Benzyl-3,4-dihydro-6-(trifluoromethyl)-2H-1,2,4-benzothiadiazine-7-sulphonamide 1,1-dioxide | Generator | | 6-Trifluoromethyl-3-benzyl-7-sulphamyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide | Generator | | Bendroflumethazide | HMDB | | Bendrofumethiazide | HMDB | | Benzydroflumethiazide | HMDB | | Benzylhydroflumethiazide | HMDB | | BHFT | HMDB | | Bendroflumethiazide apothecon brand | MeSH, HMDB | | Bristol-myers squibb brand OF bendroflumethiazide | MeSH, HMDB | | DDSA brand OF bendroflumethiazide | MeSH, HMDB | | Esberizid | MeSH, HMDB | | Naturine | MeSH, HMDB | | NeoNaClex | MeSH, HMDB | | Schaper and brümmer brand OF bendroflumethiazide | MeSH, HMDB | | Bendroflumethiazide berk brand | MeSH, HMDB | | Bendroflumethiazide ddsa brand | MeSH, HMDB | | Bendroflumethiazide goldshield brand | MeSH, HMDB | | Bendroflumethiazide leo brand | MeSH, HMDB | | Bristol myers squibb brand OF bendroflumethiazide | MeSH, HMDB | | Urizid | MeSH, HMDB | | Aprinox | MeSH, HMDB | | Bendroflumethiazide protea brand | MeSH, HMDB | | Benzide | MeSH, HMDB | | BenzideM | MeSH, HMDB | | Berkozide | MeSH, HMDB | | leo Brand OF bendroflumethiazide | MeSH, HMDB | | neo NaClex | MeSH, HMDB | | Protea brand OF bendroflumethiazide | MeSH, HMDB | | Apothecon brand OF bendroflumethiazide | MeSH, HMDB | | Bendroflumethiazide knoll brand | MeSH, HMDB | | Benzide m | MeSH, HMDB | | Benzide-m | MeSH, HMDB | | Berk brand OF bendroflumethiazide | MeSH, HMDB | | Centyl | MeSH, HMDB | | Goldshield brand OF bendroflumethiazide | MeSH, HMDB | | Knoll brand OF bendroflumethiazide | MeSH, HMDB | | neo-NaClex | MeSH, HMDB | | Pluryl | MeSH, HMDB |
|
|---|
| Chemical Formula | C15H14F3N3O4S2 |
|---|
| Average Molecular Mass | 421.415 g/mol |
|---|
| Monoisotopic Mass | 421.038 g/mol |
|---|
| CAS Registry Number | 73-48-3 |
|---|
| IUPAC Name | 3-benzyl-1,1-dioxo-6-(trifluoromethyl)-3,4-dihydro-2H-1λ⁶,2,4-benzothiadiazine-7-sulfonamide |
|---|
| Traditional Name | bendroflumethiazide |
|---|
| SMILES | NS(=O)(=O)C1=CC2=C(NC(CC3=CC=CC=C3)NS2(=O)=O)C=C1C(F)(F)F |
|---|
| InChI Identifier | InChI=1S/C15H14F3N3O4S2/c16-15(17,18)10-7-11-13(8-12(10)26(19,22)23)27(24,25)21-14(20-11)6-9-4-2-1-3-5-9/h1-5,7-8,14,20-21H,6H2,(H2,19,22,23) |
|---|
| InChI Key | HDWIHXWEUNVBIY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2,4-benzothiadiazine-1,1-dioxides. These are aromatic heterocyclic compounds containing a 1,2,4-benzothiadiazine ring system with two S=O bonds at the 1-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Thiadiazines |
|---|
| Sub Class | Benzothiadiazines |
|---|
| Direct Parent | 1,2,4-benzothiadiazine-1,1-dioxides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2,4-benzothiadiazine-1,1-dioxide
- Secondary aliphatic/aromatic amine
- Monocyclic benzene moiety
- Benzenoid
- Organosulfonic acid amide
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Aminosulfonyl compound
- Sulfonyl
- Secondary amine
- Azacycle
- Alkyl fluoride
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organosulfur compound
- Organonitrogen compound
- Organofluoride
- Organohalogen compound
- Organic nitrogen compound
- Amine
- Alkyl halide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002f-9256200000-2f09810d08c3b35be5f0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-00di-0353900000-1c8856fba9af1de07f87 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-00di-0353900000-1c8856fba9af1de07f87 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-044i-3891000000-67bb3f0457a6200bf63e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0001900000-1c849cc4bcd762034d79 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fkc-2418900000-25933e874d81c814b797 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0076-9266000000-3bb75436e40cf56f78f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1004900000-f3cae66d652083651bc3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-5917700000-851542f7279cbf5f3d05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-9000000000-6995f6142329f7f31e6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000900000-3443d3c30769b641d1ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-1000900000-c68d28cd629dd559373a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00fs-7394100000-fb480049aa3b9433389a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000900000-4fe27d3625e4e1d204f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-5000900000-806a662f295d40b18d5a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-3fb47aed873a01dbd887 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00030040 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Bendroflumethiazide |
|---|
| Chemspider ID | 2225 |
|---|
| ChEBI ID | 3013 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C07758 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|