| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:37:27 UTC |
|---|
| Update Date | 2016-11-09 01:15:26 UTC |
|---|
| Accession Number | CHEM016575 |
|---|
| Identification |
|---|
| Common Name | Azatadine |
|---|
| Class | Small Molecule |
|---|
| Description | Antihistamines such as azatadine appear to compete with histamine for histamine H1- receptor sites on effector cells. The antihistamines antagonize those pharmacological effects of histamine which are mediated through activation of H1- receptor sites and thereby reduce the intensity of allergic reactions and tissue injury response involving histamine release. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
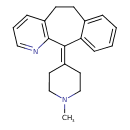
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 11-(1-Methyl-4-piperidinylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine | ChEBI | | 6,11-Dihydro-11-(1-methyl-4-piperidylidene)-5H-benzo(5,6)cyclohepta(1,2-b)pyridine | ChEBI | | Azatadina | ChEBI | | Azatadinum | ChEBI | | Azatadine maleate | HMDB | | Azatidine | HMDB | | Idulian | HMDB | | Juste brand OF azatadine maleate | HMDB | | Lergocil | HMDB | | Schering-plough brand OF azatadine maleate | HMDB | | Optimine | HMDB | | Schering brand OF azatadine maleate | HMDB | | Idulamine | HMDB | | Key brand OF azatadine maleate | HMDB | | Zadine | HMDB |
|
|---|
| Chemical Formula | C20H22N2 |
|---|
| Average Molecular Mass | 290.402 g/mol |
|---|
| Monoisotopic Mass | 290.178 g/mol |
|---|
| CAS Registry Number | 3964-81-6 |
|---|
| IUPAC Name | 2-(1-methylpiperidin-4-ylidene)-4-azatricyclo[9.4.0.0³,⁸]pentadeca-1(15),3(8),4,6,11,13-hexaene |
|---|
| Traditional Name | azatadine |
|---|
| SMILES | CN1CCC(CC1)=C1C2=CC=CC=C2CCC2=C1N=CC=C2 |
|---|
| InChI Identifier | InChI=1S/C20H22N2/c1-22-13-10-16(11-14-22)19-18-7-3-2-5-15(18)8-9-17-6-4-12-21-20(17)19/h2-7,12H,8-11,13-14H2,1H3 |
|---|
| InChI Key | SEBMTIQKRHYNIT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzocycloheptapyridines. These are aromatic compounds containing a benzene ring and a pyridine ring fused to a seven membered carbocycle. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzocycloheptapyridines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzocycloheptapyridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzocycloheptapyridine
- Benzenoid
- Pyridine
- Piperidine
- Heteroaromatic compound
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-003r-1290000000-ca0638c59dea202e717c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-584a39441c3649ae12e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ox-1390000000-c5c0b825382fa6f3cd23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02u0-8290000000-f51daaefc04a58d431d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-5ab5f6f4dcf98a4568cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1190000000-06b890698214485f4bc3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0229-5190000000-3d41734a0c4ce9ac0940 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-897430b0af8c07f94473 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0090000000-67eb5d7b0ea595cdb4c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03e9-0190000000-984c09ddd4091f9eed65 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-5f28f51a8f4e4bc977e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0190000000-cc7ee4d2f2c29b712a28 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-015i-0090000000-9faba0c5bacb0d9e73ca | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00719 |
|---|
| HMDB ID | HMDB0014857 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Azatadine |
|---|
| Chemspider ID | 18709 |
|---|
| ChEBI ID | 2946 |
|---|
| PubChem Compound ID | 19861 |
|---|
| Kegg Compound ID | C07774 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|