| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:37:14 UTC |
|---|
| Update Date | 2016-11-09 01:15:26 UTC |
|---|
| Accession Number | CHEM016567 |
|---|
| Identification |
|---|
| Common Name | Anisindione |
|---|
| Class | Small Molecule |
|---|
| Description | Anisindione is a synthetic anticoagulant and an indanedione derivative. Its anticoagulant action is mediated through the inhibition of the vitamin K-mediated gamma-carboxylation of precursor proteins that are critical in forming the formation of active procoagulation factors II, VII, IX, and X, as well as the anticoagulant proteins C and S, in the liver. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
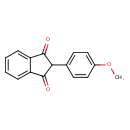
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(4-Methoxyphenyl)-1H-indene-1,3(2H)-dione | ChEBI | | 2-(4-Methoxyphenyl)indan-1,3-dione | ChEBI | | 2-(p-Methoxyphenyl)-1,3-indandione | ChEBI | | 2-(p-Methoxyphenyl)indane-1,3-dione | ChEBI | | 2-p-Anisyl-1,3-indandione | ChEBI | | 2-Para-anisyl-1,3-indandione | ChEBI | | Anisin indandione | ChEBI | | Anisindiona | ChEBI | | Anisindionum | ChEBI | | Miradon | Kegg |
|
|---|
| Chemical Formula | C16H12O3 |
|---|
| Average Molecular Mass | 252.265 g/mol |
|---|
| Monoisotopic Mass | 252.079 g/mol |
|---|
| CAS Registry Number | 117-37-3 |
|---|
| IUPAC Name | 2-(4-methoxyphenyl)-2,3-dihydro-1H-indene-1,3-dione |
|---|
| Traditional Name | anisindione |
|---|
| SMILES | COC1=CC=C(C=C1)C1C(=O)C2=CC=CC=C2C1=O |
|---|
| InChI Identifier | InChI=1S/C16H12O3/c1-19-11-8-6-10(7-9-11)14-15(17)12-4-2-3-5-13(12)16(14)18/h2-9,14H,1H3 |
|---|
| InChI Key | XRCFXMGQEVUZFC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indanediones. Indanediones are compounds containing an indane ring bearing two ketone groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Indanes |
|---|
| Sub Class | Indanones |
|---|
| Direct Parent | Indanediones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Indanedione
- Phenoxy compound
- Anisole
- Methoxybenzene
- Aryl alkyl ketone
- Aryl ketone
- Phenol ether
- Alkyl aryl ether
- 1,3-diketone
- Monocyclic benzene moiety
- 1,3-dicarbonyl compound
- Ketone
- Ether
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Organic oxide
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0pi0-2970000000-3072f4dff3d83d6798e0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0090000000-9a00a2012741ee8d529d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0290000000-ae6d022536ede61e9a68 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfr-5970000000-697cb6e17075d45c94f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-1e24bd40cb0266b81059 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-db4a7df8621482e26e27 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-1490000000-17630a8bb2f337db9f2c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0090000000-8d91e33e4298322ba53d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0690000000-5f01d86cb0ff3819b608 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9510000000-c5661b94b65978419e91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-aedd17caff57899b7eff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-de3608db6e681e51eb8b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0532-0940000000-2d549322c6ad1c2373b4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01125 |
|---|
| HMDB ID | HMDB0015257 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Anisindione |
|---|
| Chemspider ID | 2112 |
|---|
| ChEBI ID | 133809 |
|---|
| PubChem Compound ID | 2197 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|