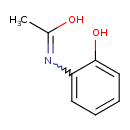
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-3900000000-4d9102b66c05fcde72e4 | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00e9-8970000000-90bf32dc54b96f2ac02c | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-0w29-0900000000-af77e021f255b3b8fac0 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0w29-0900000000-fef297ff805f8db233f1 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Negative | splash10-0a4i-0900000000-d43684d0a48bdabe138e | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-03di-1900000000-f3793f07afce760fff06 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-03di-0900000000-c9d1b55ac09e031197f2 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Negative | splash10-0a4i-0900000000-75bb3fca4fa6220b174c | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-0a4i-0900000000-f6d6bd9ad3bc874fabd5 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-0a4i-0900000000-ab0e2f7e82eb134f3b0a | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-03xr-9700000000-4704f18c2b30e7d4b4f1 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-03di-4900000000-d59f856eb2831dfe2eef | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Negative | splash10-0a4i-0900000000-149c09f49abe5f9581f2 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-0a4i-0900000000-1876114b794275814876 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0zfr-0900000000-fc4c73f9baccb46248d8 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1900000000-ab81b53931ce6ab0f825 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9500000000-15d739304f54ad540f65 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-1086be7b11354178c768 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-2900000000-d009c4036acd34f5b83a | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9200000000-90fe96bd72602d40b92b | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-b065bf14e56bbaed60a1 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-2bbceee473f22d15a64d | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-6900000000-4a2a4932ca605186545a | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-d3551e9940af66c88a11 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-2900000000-3a0fa5be1ac01e1e12c4 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0w30-9100000000-7088c0fee50a79c98570 | Spectrum |