| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:33:35 UTC |
|---|
| Update Date | 2016-11-09 01:15:24 UTC |
|---|
| Accession Number | CHEM016479 |
|---|
| Identification |
|---|
| Common Name | Thiopental sodium |
|---|
| Class | Small Molecule |
|---|
| Description | An organic sodium salt having thiopental(1-) as the counter-ion. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
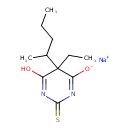
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Monosodium 5-ethyl-5-(1-methylbutyl) thiobarbiturate | ChEBI | | Penthiobarbital sodium | ChEBI | | Sodium (+-)-5-ethyl-5-(1-methylbutyl)-2-thiobarbiturate | ChEBI | | Sodium 5-ethyl-5-(1-methylbutyl)-2-thiobarbiturate | ChEBI | | Sodium pentothal | ChEBI | | Sodium pentothiobarbital | ChEBI | | Sodium thiopental | ChEBI | | Sodium thiopentobarbital | ChEBI | | Sodium thiopentone | ChEBI | | Thiopental sodique | ChEBI | | Thiopentalum natricum | ChEBI | | Thiopentone sodium | ChEBI | | Tiopental sodico | ChEBI | | Pentothal | Kegg | | Monosodium 5-ethyl-5-(1-methylbutyl) thiobarbituric acid | Generator | | Sodium (+-)-5-ethyl-5-(1-methylbutyl)-2-thiobarbituric acid | Generator | | Sodium 5-ethyl-5-(1-methylbutyl)-2-thiobarbituric acid | Generator | | Sodium;5-ethyl-5-pentan-2-yl-2-sulphanylidenepyrimidin-3-ide-4,6-dione | Generator | | Monosodium 5-ethyl-5-(1-methylbutyl) thiobarbitate | Generator | | Monosodium 5-ethyl-5-(1-methylbutyl) thiobarbitic acid | Generator | | Sodium (+-)-5-ethyl-5-(1-methylbutyl)-2-thiobarbitate | Generator | | Sodium (+-)-5-ethyl-5-(1-methylbutyl)-2-thiobarbitic acid | Generator | | Sodium 5-ethyl-5-(1-methylbutyl)-2-thiobarbitate | Generator | | Sodium 5-ethyl-5-(1-methylbutyl)-2-thiobarbitic acid | Generator | | Abbott brand OF thiopental sodium | MeSH | | Pentothal sodico | MeSH | | Sodipental | MeSH | | Thiopental sodium | MeSH | | Thiopentone | MeSH | | Nesdonal | MeSH | | Pharmtech brand OF thiopental sodium | MeSH | | Thiopentobarbital | MeSH | | Merial brand OF thiopental sodium | MeSH | | Rhone merieux brand OF thiopental sodium | MeSH | | Thionembutal | MeSH | | Tiobarbital braun | MeSH | | Pisa brand OF thiopental sodium | MeSH | | Thiomebumal | MeSH | | Trapanal | MeSH | | Bomathal | MeSH | | Braun brand OF thiopental sodium | MeSH | | Thiopental | MeSH | | Altana pharma brand OF thiopental sodium | MeSH | | Nycomed brand OF thiopental sodium | MeSH | | Penthiobarbital | MeSH | | Thiopental nycomed | MeSH |
|
|---|
| Chemical Formula | C11H17N2NaO2S |
|---|
| Average Molecular Mass | 264.320 g/mol |
|---|
| Monoisotopic Mass | 264.091 g/mol |
|---|
| CAS Registry Number | 71-73-8 |
|---|
| IUPAC Name | sodium 5-ethyl-6-hydroxy-5-(pentan-2-yl)-2-sulfanylidene-2,5-dihydropyrimidin-4-olate |
|---|
| Traditional Name | sodium 5-ethyl-6-hydroxy-5-(pentan-2-yl)-2-sulfanylidenepyrimidin-4-olate |
|---|
| SMILES | [Na+].CCCC(C)C1(CC)C(O)=NC(=S)N=C1[O-] |
|---|
| InChI Identifier | InChI=1S/C11H18N2O2S.Na/c1-4-6-7(3)11(5-2)8(14)12-10(16)13-9(11)15;/h7H,4-6H2,1-3H3,(H2,12,13,14,15,16);/q;+1/p-1 |
|---|
| InChI Key | AWLILQARPMWUHA-UHFFFAOYSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiobarbituric acid derivatives. These are organic compounds containing a 2-thioxodihydropyrimidine-4,6(1H,5H)-dione skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Thiobarbituric acid derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiobarbiturate
- 1,3-diazinane
- Carboxylic acid derivative
- Carbene-type 1,3-dipolar compound
- Organic 1,3-dipolar compound
- Organic alkali metal salt
- Azacycle
- Organic sodium salt
- Organic salt
- Carbonyl group
- Organic oxygen compound
- Organosulfur compound
- Organooxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-7190000000-281e60225acc2763f61a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05mo-7490000000-472d45b998d38154fab9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9000000000-4872d3454b31830af416 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-2490000000-a9da66eb272cf544bcd6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-9b31aadd0212f976f3ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-1a3b06bf74cac1e928db | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT001409 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Thiopental |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 9561 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|