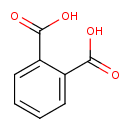
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0002-0910000000-13ae6bbb9f0d5dcc636c | Spectrum |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0zor-9500000000-e5ec33e3312a4a87ea39 | Spectrum |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0002-0910000000-b0d922c04c2f5dd6e4c7 | Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0002-0910000000-13ae6bbb9f0d5dcc636c | Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0002-0900000000-9308910720abd784ba33 | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014j-5900000000-68084b3e65fcd1cea856 | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-8390000000-d238ce57194d1385d919 | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0002-0900000000-0f913d45c1daa3650138 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00kf-9400000000-ba393489337831b167f0 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-014i-9000000000-998fd73fa45b918fbbd4 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-014i-0900000000-473374d725e62e2c60ef | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-00fr-4900000000-863cf771373d1802f7d0 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-004i-9200000000-acf56d82f3969fd63b3b | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-004i-9100000000-c03297cff4e455fc9a56 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-00di-9000000000-88a6558d781ff1f9c6c5 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-014i-0900000000-473374d725e62e2c60ef | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-00fr-4900000000-863cf771373d1802f7d0 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-004i-9200000000-9b673ed6e8d6d73b74b4 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-004i-9100000000-c03297cff4e455fc9a56 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-00di-9000000000-88a6558d781ff1f9c6c5 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-79444e6f1e1cad82c9af | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-00fr-7900000000-69919220fe286719b154 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-f8cd37ad5a7de3510c1c | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0002-0900000000-0bf84650c197f00d2d4b | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-00di-0900000000-9e9de7b1b9755cbc0c83 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-00di-0900000000-17c7a2e06ddf58f100a3 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-544e377d1efe0d8040f2 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-0900000000-88ea8f2e083f769b4ecc | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-6900000000-f4a09894e4cd81ff485e | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-e9d3605c3e0f232735dc | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00xr-2900000000-f0bd93b089cec9e1caf2 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00b9-9700000000-699743d9f7786056612c | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |