| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:28:01 UTC |
|---|
| Update Date | 2016-11-09 01:15:22 UTC |
|---|
| Accession Number | CHEM016336 |
|---|
| Identification |
|---|
| Common Name | Levobunolol hydrochloride |
|---|
| Class | Small Molecule |
|---|
| Description | A hydrochloride obtained by combining equimolar amounts of levobunolol and hydrochloric acid. A non-selective beta-adrenergic antagonist used for treatment of glaucoma. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
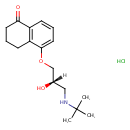
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-3,4-Dihydro-5-(3-(tert-butylamino)-2-hydroxypropoxy)-1(2H)-naphthalenone hydrochloride | ChEBI | | (-)-5-(3-(Tert-butylamino)-2-hydroxypropoxy)-3,4-dihydro-1(2H)-naphthalenone hydrochloride | ChEBI | | (2S)-N-(Tert-butyl)-2-hydroxy-3-[(5-oxo-5,6,7,8-tetrahydronaphthalen-1-yl)oxy]propan-1-aminium chloride | ChEBI | | Betagan | ChEBI | | Levobunolol HCL | ChEBI | | Levobunolol monohydrochloride | ChEBI | | Novo-levobunolol | MeSH | | Apo levobunolol | MeSH | | Apo-levobunolol | MeSH | | ApoLevobunolol | MeSH | | Levobunolol | MeSH | | Vistagan | MeSH | | Ultracortenol | MeSH | | Ratio-levobunolol | MeSH | | Novo levobunolol | MeSH | | PMS-Levobunolol | MeSH | | AKBeta | MeSH | | PMS Levobunolol | MeSH | | PMSLevobunolol | MeSH | | Ratio levobunolol | MeSH | | NovoLevobunolol | MeSH | | Allergan brand OF levobunolol hydrochloride | MeSH | | Ciba vision brand OF levobunolol hydrochloride | MeSH | | Novopharm brand OF levobunolol hydrochloride | MeSH | | Ratiopharm brand OF levobunolol hydrochloride | MeSH | | Apotex brand OF levobunolol hydrochloride | MeSH | | Akorn brand OF levobunolol hydrochloride | MeSH | | Pharm allergan brand OF levobunolol hydrochloride | MeSH | | Pharm-allergan brand OF levobunolol hydrochloride | MeSH | | Pharmascience brand OF levobunolol hydrochloride | MeSH | | Levobunolol hydrochloride | MeSH |
|
|---|
| Chemical Formula | C17H26ClNO3 |
|---|
| Average Molecular Mass | 327.846 g/mol |
|---|
| Monoisotopic Mass | 327.160 g/mol |
|---|
| CAS Registry Number | 27912-14-7 |
|---|
| IUPAC Name | 5-[(2S)-3-(tert-butylamino)-2-hydroxypropoxy]-1,2,3,4-tetrahydronaphthalen-1-one hydrochloride |
|---|
| Traditional Name | akβ hydrochloride |
|---|
| SMILES | Cl.[H][C@](O)(CNC(C)(C)C)COC1=CC=CC2=C1CCCC2=O |
|---|
| InChI Identifier | InChI=1S/C17H25NO3.ClH/c1-17(2,3)18-10-12(19)11-21-16-9-5-6-13-14(16)7-4-8-15(13)20;/h5-6,9,12,18-19H,4,7-8,10-11H2,1-3H3;1H/t12-;/m0./s1 |
|---|
| InChI Key | DNTDOBSIBZKFCP-YDALLXLXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetralins. These are polycyclic aromatic compounds containing a tetralin moiety, which consists of a benzene fused to a cyclohexane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Tetralins |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tetralins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetralin
- Aryl ketone
- Aryl alkyl ketone
- Alkyl aryl ether
- 1,2-aminoalcohol
- Ketone
- Secondary alcohol
- Secondary aliphatic amine
- Ether
- Secondary amine
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Alcohol
- Organic nitrogen compound
- Amine
- Organic zwitterion
- Organic chloride salt
- Organic salt
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-000b-2910000000-46a1e94d122d95727d9a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-000b-2910000000-46a1e94d122d95727d9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0009000000-9d9617b8cc871fd4e8a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0009000000-9d9617b8cc871fd4e8a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-0009000000-9d9617b8cc871fd4e8a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-bc7e18785d84c849debc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0009000000-bc7e18785d84c849debc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0009000000-bc7e18785d84c849debc | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT000251 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Levobunolol |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 6439 |
|---|
| PubChem Compound ID | 441415 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|