| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:27:54 UTC |
|---|
| Update Date | 2016-11-09 01:15:22 UTC |
|---|
| Accession Number | CHEM016332 |
|---|
| Identification |
|---|
| Common Name | Isomalt |
|---|
| Class | Small Molecule |
|---|
| Description | Isomalt is a sugar substitute, a type of sugar alcohol used primarily for its sugar-like physical properties. It has little to no impact on blood sugar levels, and does not stimulate the release of insulin. It also does not promote tooth decay, i.e. is tooth-friendly. Its energy value is 2 kcal/g, half that of sugars. However, like most sugar alcohols, it carries a risk of gastric distress, including flatulence and diarrhea, when consumed in large quantities (above about 20-30 g per day). Isomalt may prove upsetting to the intestinal tract because it is incompletely absorbed in the small intestine, and when polyols pass into the large intestine, they can cause osmotically induced diarrhea and stimulate the gut flora, causing flatulence. As with other dietary fibers, regular consumption of isomalt can lead to desensitisation, decreasing the risk of intestinal upset. Isomalt can be blended with high-intensity sweeteners such as sucralose, giving a mixture that has the same sweetness as sugar.
Isomalt is an equimolar mixture of two mutually diastereomeric disaccharides, each composed of two sugars: glucose and mannitol (α-D-glucopyranosido-1,6-mannitol) and also glucose and sorbitol (α-D-glucopyranosido-1,6-sorbitol). Complete hydrolysis of isomalt yields glucose (50%), sorbitol (25%), and mannitol (25%). It is an odorless, white, crystalline substance containing about 5% water of crystallisation. Isomalt has a minimal cooling effect (positive heat of solution), lower than many other sugar alcohols, in particular, xylitol and erythritol.
Isomalt is manufactured in a two-stage process in which sucrose is first transformed into isomaltulose, a reducing disaccharide (6-O-α-D-glucopyranosido-D-fructose). The isomaltulose is then hydrogenated, using a Raney nickel catalyst. The final product — isomalt — is an equimolar composition of 6-O-α-D-glucopyranosido-D-sorbitol (1,6-GPS) and 1-O-α-D-glucopyranosido-D-mannitol-dihydrate (1,1-GPM-dihydrate).
Isomalt has been approved for use in the United States since 1990. It is also permitted for use in Australia, New Zealand, Canada, Mexico, Iran, the European Union, and other countries. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| D-Glucitol, 6-O-alpha-D-glucopyranosyl-, mixt. with 1-O-alpha-D-glucopyranosyl-D-mannitol | MeSH | | D-Glucitol, 6-O-alpha-D-glucopyranosyl-, mixture with 1-O-alpha-D-glucopyranosyl-D-mannitol | MeSH | | BAY I 3930 | ChEMBL |
|
|---|
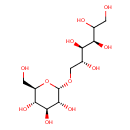
| Chemical Formula | C12H24O11 |
|---|
| Average Molecular Mass | 344.312 g/mol |
|---|
| Monoisotopic Mass | 344.132 g/mol |
|---|
| CAS Registry Number | 64519-82-0 |
|---|
| IUPAC Name | (3R,4R,5R)-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}hexane-1,2,3,4,5-pentol |
|---|
| Traditional Name | isomalt |
|---|
| SMILES | [H]C(O)(CO)[C@@]([H])(O)[C@]([H])(O)[C@]([H])(O)CO[C@@]1([H])O[C@]([H])(CO)[C@@]([H])(O)[C@]([H])(O)[C@@]1([H])O |
|---|
| InChI Identifier | InChI=1S/C12H24O11/c13-1-4(15)7(17)8(18)5(16)3-22-12-11(21)10(20)9(19)6(2-14)23-12/h4-21H,1-3H2/t4?,5-,6-,7-,8-,9-,10+,11-,12+/m1/s1 |
|---|
| InChI Key | SERLAGPUMNYUCK-BLEZHGCXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty acyl glycosides of mono- and disaccharides. Fatty acyl glycosides of mono- and disaccharides are compounds composed of a mono- or disaccharide moiety linked to one hydroxyl group of a fatty alcohol or of a phosphorylated alcohol (phosphoprenols), a hydroxy fatty acid or to one carboxyl group of a fatty acid (ester linkage) or to an amino alcohol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl glycosides |
|---|
| Direct Parent | Fatty acyl glycosides of mono- and disaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acyl glycoside of mono- or disaccharide
- Alkyl glycoside
- Glycosyl compound
- O-glycosyl compound
- Fatty alcohol
- Sugar alcohol
- Monosaccharide
- Oxane
- Secondary alcohol
- Acetal
- Organoheterocyclic compound
- Oxacycle
- Polyol
- Organooxygen compound
- Primary alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (9 TMS) | splash10-0uxr-0961000000-9e1d9919fd01f1a276bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-2429000000-566fa40afdfb8242cd09 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-9531000000-2a65651e8e0fa8de4797 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dl-9420000000-b9f23579781370da5e56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-07bo-7952000000-bba4691868723e7ed321 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08p0-9641000000-ab2958743c71db68d10b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0lxx-9510000000-2e7d08f239be123106ed | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Isomalt |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 3034828 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|