| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:25:18 UTC |
|---|
| Update Date | 2016-11-09 01:15:22 UTC |
|---|
| Accession Number | CHEM016269 |
|---|
| Identification |
|---|
| Common Name | Colcemid |
|---|
| Class | Small Molecule |
|---|
| Description | A secondary amino compound that is (S)-colchicine in which the N-acetyl group is replaced by an N-methyl group. Isolable from the autumn crocus, Colchicum autumnale, it is less toxic than colchicine and is used as an antineoplastic. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
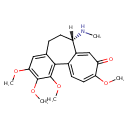
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-Colchamine | ChEBI | | Colcemid | ChEBI | | Demecolcina | ChEBI | | Demecolcinum | ChEBI | | N-Deacetyl-N-methylcolchicine | ChEBI | | N-Desacetyl-N-methylcolchicine | ChEBI | | N-Methyl-N-deacetylcolchicine | ChEBI | | N-Methyl-N-desacetylcolchicine | ChEBI | | Reichstein's F | ChEBI | | Santavy's substance F | ChEBI | | Demecolcine | ChEBI | | Demecolcine, (+-)-isomer | MeSH | | Colcemide | MeSH | | Colchamine | MeSH |
|
|---|
| Chemical Formula | C21H25NO5 |
|---|
| Average Molecular Mass | 371.433 g/mol |
|---|
| Monoisotopic Mass | 371.173 g/mol |
|---|
| CAS Registry Number | 477-30-5 |
|---|
| IUPAC Name | (10S)-3,4,5,14-tetramethoxy-10-(methylamino)tricyclo[9.5.0.0²,⁷]hexadeca-1(16),2,4,6,11,14-hexaen-13-one |
|---|
| Traditional Name | colcemid |
|---|
| SMILES | [H][C@@]1(CCC2=CC(OC)=C(OC)C(OC)=C2C2=CC=C(OC)C(=O)C=C12)NC |
|---|
| InChI Identifier | InChI=1S/C21H25NO5/c1-22-15-8-6-12-10-18(25-3)20(26-4)21(27-5)19(12)13-7-9-17(24-2)16(23)11-14(13)15/h7,9-11,15,22H,6,8H2,1-5H3/t15-/m0/s1 |
|---|
| InChI Key | NNJPGOLRFBJNIW-HNNXBMFYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tropones. Tropones are compounds containing a tropone ring, which is a cycloheptatrienone ring bearing a ketone group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Hydrocarbon derivatives |
|---|
| Class | Tropones |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tropones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Anisole
- Tropone
- Alkyl aryl ether
- Aralkylamine
- Benzenoid
- Cyclic ketone
- Secondary aliphatic amine
- Ether
- Secondary amine
- Organooxygen compound
- Organonitrogen compound
- Amine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-03dj-1492000000-9f8537d47622417c429d | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-03dj-1492000000-9f8537d47622417c429d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009000000-1f6ddc8c3363ad6a5e35 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-0009000000-ae75b1400501b9297fd7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056r-0194000000-6f0baeb765b5d92eb6df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-ed8c1a1821d8c5705282 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fk9-0009000000-aa4e7b5666cfdcfd354d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0i7l-3092000000-52f10777fa69e59d89a0 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB13318 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00027138 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Demecolcine |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 4393 |
|---|
| PubChem Compound ID | 220401 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|