| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-20 16:12:17 UTC |
|---|
| Update Date | 2016-11-09 01:15:19 UTC |
|---|
| Accession Number | CHEM016043 |
|---|
| Identification |
|---|
| Common Name | Vinblastine sulfate |
|---|
| Class | Small Molecule |
|---|
| Description | Antitumor alkaloid isolated from Vinca rosea. (Merck, 11th ed.) |
|---|
| Contaminant Sources | - IARC Carcinogens Group 3
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
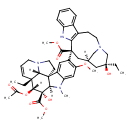
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Velban | ChEMBL, MeSH | | [3H]-Vinblastine | ChEMBL | | Gry brand OF vinblastine sulfate | MeSH | | Hexal brand OF vinblastine sulfate | MeSH | | Lilly brand OF vinblastine sulfate | MeSH | | Velbe | MeSH | | EG labo brand OF vinblastine sulfate | MeSH | | Faulding brand OF vinblastine sulfate | MeSH | | Lemblastine | MeSH | | Vinblastinsulfat-gry | MeSH | | Vincaleukoblastine | MeSH | | Vinblastin hexal | MeSH | | Vinblastina lilly | MeSH | | Cell pharm brand OF vinblastine sulfate | MeSH | | Gastrozepin brand OF vinblastine sulfate | MeSH | | Lemery brand OF vinblastine sulfate | MeSH | | Sulfate, vinblastine | MeSH | | Vinblastine sulfate | MeSH | | Cellblastin | MeSH |
|
|---|
| Chemical Formula | C46H58N4O9 |
|---|
| Average Molecular Mass | 810.974 g/mol |
|---|
| Monoisotopic Mass | 810.420 g/mol |
|---|
| CAS Registry Number | 143-67-9 |
|---|
| IUPAC Name | methyl (1R,9R,10S,11R,12R,19R)-11-(acetyloxy)-12-ethyl-4-[(13S,15R,17S)-17-ethyl-17-hydroxy-13-(methoxycarbonyl)-1,11-diazatetracyclo[13.3.1.0⁴,¹².0⁵,¹⁰]nonadeca-4(12),5,7,9-tetraen-13-yl]-10-hydroxy-5-methoxy-8-methyl-8,16-diazapentacyclo[10.6.1.0¹,⁹.0²,⁷.0¹⁶,¹⁹]nonadeca-2,4,6,13-tetraene-10-carboxylate |
|---|
| Traditional Name | vinblastine |
|---|
| SMILES | [H][C@@]12N(C)C3=CC(OC)=C(C=C3[C@@]11CCN3CC=C[C@](CC)([C@@]13[H])[C@@]([H])(OC(C)=O)[C@]2(O)C(=O)OC)[C@]1(C[C@@]2([H])CN(C[C@](O)(CC)C2)CCC2=C1NC1=CC=CC=C21)C(=O)OC |
|---|
| InChI Identifier | InChI=1S/C46H58N4O9/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3/t28-,37-,38+,39+,42-,43+,44+,45-,46-/m0/s1 |
|---|
| InChI Key | JXLYSJRDGCGARV-CFWMRBGOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as vinca alkaloids. These are alkaloids with a dimeric chemical structure composed of an indole nucleus (catharanthine), and a dihydroindole nucleus (vindoline), joined together. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Vinca alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Vinca alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Vinca alkaloid skeleton
- Carbazole
- 3-alkylindole
- Tricarboxylic acid or derivatives
- Indole or derivatives
- Indole
- Tertiary aliphatic/aromatic amine
- Dialkylarylamine
- Anisole
- Aralkylamine
- Alkyl aryl ether
- Piperidine
- Benzenoid
- N-alkylpyrrolidine
- Methyl ester
- Tertiary alcohol
- Pyrrole
- Heteroaromatic compound
- Pyrrolidine
- Cyclic alcohol
- 1,2-aminoalcohol
- Amino acid or derivatives
- Carboxylic acid ester
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Ether
- Carboxylic acid derivative
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxide
- Organic nitrogen compound
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ikc-0000000910-d1cb68f2685afb162acc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uec-0000000900-d971ad5e494178026027 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-2200003900-b38c610455defefb74ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-2004000940-18f9743c5af790ad89f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-0009000200-0c81f1924aad5a0d0289 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9015000800-316693e83321d70e4472 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00570 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Vinblastine |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 13342 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|