| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-20 16:10:41 UTC |
|---|
| Update Date | 2016-11-09 01:15:19 UTC |
|---|
| Accession Number | CHEM016013 |
|---|
| Identification |
|---|
| Common Name | Furazolidone |
|---|
| Class | Small Molecule |
|---|
| Description | Furazolidone is a nitrofuran derivative with antiprotozoal and antibacterial activity. Furazolidone has been shown to exhibit antibiotic and anti-microbial functions (PMID 1476092, 6651278). Furazolidone is also used as a poultry food additive. It is marketed by Roberts Laboratories under the brand name Furazolidone and by GlaxoSmithKline as Dependal-M. Furazolidone has a broad antibacterial spectrum covering the majority of gastrointestinal tract pathogens including E. coli, staphylococci, Salmonella, Shigella, Proteus, Aerobacter aerogenes, Vibrio cholerae and Giardia lamblia. Its bactericidal activity is based upon its interference with DNA replication and protein production. Furazolidone binds bacterial DNA which leads to the gradual inhibition of monoamine oxidase (From Martindale, The Extra Pharmacopoeia, 30th ed, p514). Furazolidone and its related free radical products are believed to bind DNA and induce cross-links. Bacterial DNA is particularly susceptible to this drug leading to high levels of mutations (transitions and transversions) in the bacterial chromosome. Furazolidone belongs to the family of Nitrofurans. These are compounds containing a furan ring which bears a nitro group. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
- IARC Carcinogens Group 3
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Furoxone | Kegg | | 3-(5'-Nitrofurfuralamino)-2-oxazolidone | HMDB | | 3-[(5-Nitrofurfurylidene)amino]-2-oxazolidinone | HMDB | | 3-[(5-Nitrofurfurylidene)amino]-2-oxazolidone | HMDB | | 3-[(5-Nitrofurylidene)amino]-2-oxazolidone | HMDB | | 3-{[(5-nitro-2-furanyl)methylene]amino}-2-oxazolidinone | HMDB | | 5-Nitro-N-(2-oxo-3-oxazolidinyl)-2-furanmethanimine | HMDB | | Furazolidona | HMDB | | Furazolidonum | HMDB | | FZL | HMDB | | N-(5-Nitro-2-furfurylidene)-3-amino-2-oxazolidone | HMDB | | N-(5-Nitro-2-furfurylidene)-3-aminooxazolidine-2-one | HMDB | | Nitrofurazolidone | HMDB | | Nitrofurazolidonum | HMDB | | 3-(((5-Nitro-2-furanyl)methylene)amino)-2-oxazolidinone | HMDB | | 3-((5-Nitrofurfurylidene)amino)-2-oxazolidinone | HMDB | | 3-((5-Nitrofurfurylidene)amino)-2-oxazolidone | HMDB | | 3-((5-Nitrofurfurylidine)amino)-2-oxazolidinone | HMDB | | 3-((5-Nitrofurylidene)amino)-2-oxazolidone | HMDB | | 3-(5-Nitrofurfurylideneamino)-2-oxazolidinone | HMDB | | 3-[(5-Nitrofurfurylidine)amino]-2-oxazolidinone | HMDB | | 3-[(e)-(5-Nitrofuran-2-yl)methylideneamino]-1,3-oxazolidin-2-one | HMDB | | 3-[[(5-Nitro-2-furanyl)methylene]-amino]-2-oxazolidinone | HMDB | | 3-[[(5-Nitro-2-furanyl)methylene]amino]-2-oxazolidinone | HMDB | | 3-[[(5-Nitro-2-furanyl)methylene]amino]-2-oxazolidinone, 9ci | HMDB | | Bifuron | HMDB | | Corizium | HMDB | | Coryzium | HMDB | | Diafuron | HMDB | | Enterotoxon | HMDB | | Fiurox aerosol powder | HMDB | | Furall | HMDB | | Furaxon | HMDB | | Furaxone | HMDB | | Furazolidine | HMDB | | Furazon | HMDB | | Furidon | HMDB | | Furovag | HMDB | | Furox | HMDB | | Furoxal | HMDB | | Furoxane | HMDB | | Furoxon | HMDB | | Furoxone swine mix | HMDB | | Furozolidine | HMDB | | Giardil | HMDB | | Giarlam | HMDB | | Medaron | HMDB | | Neftin | HMDB | | Nicolen | HMDB | | Nifulidone | HMDB | | Nifuran | HMDB | | Nifurazolidone | HMDB | | Nitrofuroxon | HMDB | | Optazol | HMDB | | Ortazol | HMDB | | Puradin | HMDB | | Roptazol | HMDB | | Sclaventerol | HMDB | | Tikofuran | HMDB | | Topazone | HMDB | | Trichofuron | HMDB | | Tricofuron | HMDB | | Tricoron | HMDB | | Trifurox | HMDB | | USAF ea-1 | HMDB | | Viofuragyn | HMDB | | Furazol | HMDB |
|
|---|
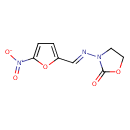
| Chemical Formula | C8H7N3O5 |
|---|
| Average Molecular Mass | 225.160 g/mol |
|---|
| Monoisotopic Mass | 225.039 g/mol |
|---|
| CAS Registry Number | 67-45-8 |
|---|
| IUPAC Name | 3-[(E)-[(5-nitrofuran-2-yl)methylidene]amino]-1,3-oxazolidin-2-one |
|---|
| Traditional Name | furazolidone |
|---|
| SMILES | [O-][N+](=O)C1=CC=C(O1)\C=N\N1CCOC1=O |
|---|
| InChI Identifier | InChI=1S/C8H7N3O5/c12-8-10(3-4-15-8)9-5-6-1-2-7(16-6)11(13)14/h1-2,5H,3-4H2/b9-5+ |
|---|
| InChI Key | PLHJDBGFXBMTGZ-WEVVVXLNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as nitrofurans. Nitrofurans are compounds containing a furan ring which bears a nitro group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Furans |
|---|
| Sub Class | Nitrofurans |
|---|
| Direct Parent | Nitrofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Nitroaromatic compound
- 2-nitrofuran
- Oxazolidinone
- Oxazolidine
- Heteroaromatic compound
- C-nitro compound
- Carbonic acid derivative
- Organic nitro compound
- Organic oxoazanium
- Allyl-type 1,3-dipolar organic compound
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Oxacycle
- Azacycle
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002f-9610000000-ab960b06286d0ccd8c6c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-1390000000-6d4a20ff37556f08e13b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1390000000-3c18d9819c68dd1ecba9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08mj-9500000000-9e58b2b1f6fd5b9343c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-2290000000-63a56a073d7d4f4cfd09 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fk9-9670000000-ddd3d4a03c2a3bc6ab9e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fk9-9000000000-58fd85feae000f4153dd | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0014752 |
|---|
| FooDB ID | FDB000957 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Furazolidone |
|---|
| Chemspider ID | 4481255 |
|---|
| ChEBI ID | 5195 |
|---|
| PubChem Compound ID | 5323714 |
|---|
| Kegg Compound ID | C07999 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|