| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-20 14:55:59 UTC |
|---|
| Update Date | 2016-11-09 01:15:18 UTC |
|---|
| Accession Number | CHEM015982 |
|---|
| Identification |
|---|
| Common Name | Fenbutatin oxide |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
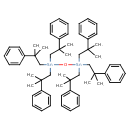
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Bis(tris(2-methyl-2-phenylpropyl)tin)oxide | ChEBI | | Bis[tris(2-methyl-2-phenylpropyl)tin]oxide | ChEBI | | Di(tri-(2,2-dimethyl-2-phenylethyl)tin)oxide | ChEBI | | Hexakis(beta,beta-dimethylphenethyl)distannoxane | ChEBI | | SD 14114 | ChEBI | | Torque | ChEBI | | Vendex | ChEBI | | Hexakis(b,b-dimethylphenethyl)distannoxane | Generator | | Hexakis(β,β-dimethylphenethyl)distannoxane | Generator | | 1,1,1,3,3,3-Hexakis(2-methyl-2-phenylpropyl)-distannoxane | HMDB | | 2-(Methyl-2-phenylpropyl)distannoxane | HMDB | | Bendex | HMDB | | Bis(trineophyltin) oxide | HMDB | | Bis(tris(2-methyl-2-phenylpropyl)tin) oxide | HMDB | | Bis(tris(beta,beta-dimethylphenethyl)tin)oxide | HMDB | | Bis[tris(2-methyl-2-phenylpropyl)tin] oxide | HMDB | | Bis[tris-(2-methyl-2-phenylpropyl)tin] oxide | HMDB | | Fenbutatin oxide | HMDB | | Fenbutatin oxide, bsi | HMDB | | Fenbutatin-oxide | HMDB | | Fenbutatin-oxyde | HMDB | | Fenylbutatin oxide | HMDB | | Fenylbutylstannium oxide | HMDB | | Hexakis | HMDB | | Hexakis (2-methyl-2-phenylpropyl)-distannoxane | HMDB | | Hexakis(2-methyl-2-phenylpropyl)-distannoxane | HMDB | | Hexakis(beta,beta-dimethylphenethyl)-distannoxane | HMDB | | Hexaneophyldistannoxane | HMDB | | Neostanox | HMDB | | Osdaran | HMDB |
|
|---|
| Chemical Formula | C60H80OSn2 |
|---|
| Average Molecular Mass | 1054.700 g/mol |
|---|
| Monoisotopic Mass | 1056.425 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | tris(2-methyl-2-phenylpropyl)({[tris(2-methyl-2-phenylpropyl)stannyl]oxy})stannane |
|---|
| Traditional Name | vendex |
|---|
| SMILES | O.[Sn].[Sn].[CH2]C(C)(C)C1=CC=CC=C1.[CH2]C(C)(C)C1=CC=CC=C1.[CH2]C(C)(C)C1=CC=CC=C1.[CH2]C(C)(C)C1=CC=CC=C1.[CH2]C(C)(C)C1=CC=CC=C1.[CH2]C(C)(C)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/6C10H13.H2O.2Sn/c6*1-10(2,3)9-7-5-4-6-8-9;;;/h6*4-8H,1H2,2-3H3;1H2;; |
|---|
| InChI Key | LVHRFSXPIDPHTC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpropanes. These are organic compounds containing a phenylpropane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylpropanes |
|---|
| Direct Parent | Phenylpropanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylpropane
- Trialkyltin
- Organic metal salt
- Organic oxygen compound
- Hydrocarbon derivative
- Organic tin salt
- Organic salt
- Organotin compound
- Organometallic compound
- Organic post-transition metal moeity
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9000000000-ef8213852ff51ef00509 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0900040000-5ccf94f609446d31a5e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9000000002-7b731e129afb37bff489 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0uxr-9000140004-6d3ec0d4f3deed4e338a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uxr-9200030004-3b88cdc69425f94aa87b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00o0-8901010203-9cdc9953c6c2e0ceee57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-6100000009-cc964504701061787362 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05nr-4900010102-4c0b88513a50531fbac7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05p9-4900000001-2871f63611403251baa4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-9000000000-4eb1a6bd8e75e989429a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uxr-8900000001-4bd12e0f0fb7d9e77c77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-2100000900-2ceef8f562f1028bacd5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031788 |
|---|
| FooDB ID | FDB008461 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10468814 |
|---|
| ChEBI ID | 39294 |
|---|
| PubChem Compound ID | 16683004 |
|---|
| Kegg Compound ID | C15435 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|