| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-20 14:52:33 UTC |
|---|
| Update Date | 2016-10-28 10:00:48 UTC |
|---|
| Accession Number | CHEM015908 |
|---|
| Identification |
|---|
| Common Name | Norpseudoephedrine |
|---|
| Class | Small Molecule |
|---|
| Description | An amphetamine in which the parent 1-phenylpropan-2-amine skeleton is substituted at position 1 with an hydroxy group. A decongestant and appetite suppressant, it is commonly used in prescription and over-the-counter cough and cold preparations. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
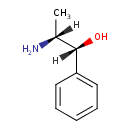
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| beta-Hydroxyamphetamine | ChEBI | | Fenilpropanolamina | ChEBI | | Norephedrine | ChEBI | | Phenylpropanolaminum | ChEBI | | PPA | ChEBI | | (+-)-Phenylpropanolamine | Kegg | | (R*,s*)-(+-)-alpha-(1-aminoethyl)benzenemethanol | Kegg | | (+-)-Norephedrine | Kegg | | Fansia | Kegg | | b-Hydroxyamphetamine | Generator | | Β-hydroxyamphetamine | Generator | | (R*,s*)-(+-)-a-(1-aminoethyl)benzenemethanol | Generator | | (R*,s*)-(+-)-α-(1-aminoethyl)benzenemethanol | Generator | | Fugoa depot | MeSH | | Norpseudoephedrine, (S-(r*,s*))-isomer | MeSH | | Fasupond | MeSH | | Exponcit | MeSH | | Norpseudoephedrine hydrochloride, (r*,s*)-isomer | MeSH | | Norpseudoephedrine sulfate (2:1), (R-(r*,r*))-isomer | MeSH | | Norpseudoephedrine, (S-(r*,r*))-isomer | MeSH | | Norpseudoephedrine hydrobromide | MeSH | | Norpseudoephedrine hydrochloride, (S-(r*,s*))-isomer | MeSH | | Cathine hydrochloride | MeSH | | Norpseudoephedrine | MeSH | | Norpseudoephedrine, (r*,s*)-isomer | MeSH | | Dexatrim | MeSH | | Hydrochloride, phenylpropanolamine | MeSH | | Triaminic DM | MeSH | | Norpseudoephedrine sulfate, (R-(r*,s*))-isomer | MeSH | | Prolamine | MeSH | | Norpseudoephedrine hydrochloride | MeSH | | Norpseudoephedrine hydrochloride, (R-(r*,r*))-isomer | MeSH | | Norpseudoephedrine tartrate, (S-(r*,r*))-(R-(r*,r*))-isomer | MeSH | | Propagest | MeSH | | Norpseudoephedrine, (R-(r*,s*))-isomer | MeSH | | Norpseudoephedrine hydrochloride, (+)-isomer | MeSH | | Norpseudoephedrine sulfate, (S-(r*,r*))-isomer | MeSH | | Norpseudoephedrine sulfate (2:1), (+)-isomer | MeSH | | Pseudonorephedrine | MeSH | | Norpseudoephedrine, (-)-isomer | MeSH | | Norpseudoephedrine, (r*,r*)-(+-)-isomer | MeSH | | (+)-Norpseudoephedrine | MeSH | | Norpseudoephedrine hydrochloride, (r*,s*)-(+-)-isomer | MeSH | | Phenylpropanolamine hydrochloride | MeSH | | Cathine | MeSH | | Norpseudoephedrine, conjugate monoacid, (R-(r*,s*))-isomer | MeSH |
|
|---|
| Chemical Formula | C9H13NO |
|---|
| Average Molecular Mass | 151.206 g/mol |
|---|
| Monoisotopic Mass | 151.100 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (1R,2R)-2-amino-1-phenylpropan-1-ol |
|---|
| Traditional Name | (1R,2R)-2-amino-1-phenylpropan-1-ol |
|---|
| SMILES | [H][C@](C)(N)[C@]([H])(O)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C9H13NO/c1-7(10)9(11)8-5-3-2-4-6-8/h2-7,9,11H,10H2,1H3/t7-,9+/m1/s1 |
|---|
| InChI Key | DLNKOYKMWOXYQA-APPZFPTMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpropanes. These are organic compounds containing a phenylpropane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylpropanes |
|---|
| Direct Parent | Phenylpropanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylpropane
- Aralkylamine
- 1,2-aminoalcohol
- Secondary alcohol
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic alcohol
- Organopnictogen compound
- Primary amine
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Amine
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-0900000000-e5a6de9f258209c42ea3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0159-0900000000-983fa1b112e4d0fc78e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-5900000000-94c2913c48414f6f2f68 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-9a4b3eaff66d145e5863 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zgi-2900000000-3c0efa3ebff61c0c1a7d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-057i-9800000000-430f9914117dd6db13e1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Phenylpropanolamine |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 8104 |
|---|
| PubChem Compound ID | 162265 |
|---|
| Kegg Compound ID | C07911 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|