| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 04:52:05 UTC |
|---|
| Update Date | 2016-11-09 01:15:14 UTC |
|---|
| Accession Number | CHEM015484 |
|---|
| Identification |
|---|
| Common Name | 3,3'4,4'-Biphenyltetramine |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the class of biphenyls that is benzidine in which one of the hydrogens ortho to each of the amino groups has been replaced by an amino group. |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
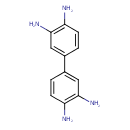
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (1,1'-Biphenyl)-3,3',4,4'-tetramine | ChEBI | | 3,3',4,4'-Biphenyltetramine | ChEBI | | 3,3',4,4'-Diphenyltetramine | ChEBI | | 3,3',4,4'-Tetraaminobiphenyl | ChEBI | | 3,3',4,4'-Tetraaminodiphenyl | ChEBI | | 3,3',4,4'-Tetraminobiphenyl | ChEBI | | 3,3'-Diaminobenzidene | ChEBI | | Biphenyl-3,3',4,4'-tetrayltetraamine | ChEBI | | DAB | ChEBI | | 3,3-Diaminobenzidine | HMDB | | Diaminobenzidine, 3,3' | HMDB | | Diaminobenzidine, 3,3 | HMDB | | 3,3' Diaminobenzidine | HMDB | | 3,3 Diaminobenzidine | HMDB | | 3,3'-Diaminobenzidine | MeSH |
|
|---|
| Chemical Formula | C12H14N4 |
|---|
| Average Molecular Mass | 214.272 g/mol |
|---|
| Monoisotopic Mass | 214.122 g/mol |
|---|
| CAS Registry Number | 91-95-2 |
|---|
| IUPAC Name | [1,1'-biphenyl]-3,3',4,4'-tetramine |
|---|
| Traditional Name | [1,1'-biphenyl]-3,3',4,4'-tetramine |
|---|
| SMILES | NC1=C(N)C=C(C=C1)C1=CC(N)=C(N)C=C1 |
|---|
| InChI Identifier | InChI=1S/C12H14N4/c13-9-3-1-7(5-11(9)15)8-2-4-10(14)12(16)6-8/h1-6H,13-16H2 |
|---|
| InChI Key | HSTOKWSFWGCZMH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 3,3'-disubstituted benzidines. These are organic compounds containing a benzidine skeleton, which is substituted only at the 3- and 3'-positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Biphenyls and derivatives |
|---|
| Direct Parent | 3,3'-disubstituted benzidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3,3'-disubstituted benzidine
- Aniline or substituted anilines
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary amine
- Organonitrogen compound
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-1970000000-e300db96232e69d8389e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0940000000-a021c5af17798249ceb6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0910000000-13cf2284a80ad04e41b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00xr-0900000000-61263896d7cde2851570 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-5ddc23864e786f6aa7f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dj-0590000000-e5a08fcfd0677816e1fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ot-3940000000-7c0e93994436f6bb32fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-93057deb8db133f12abf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ot-0950000000-f17111d6e3240924529a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000t-0900000000-e9da7f605fd51e0b65f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0290000000-4e3c6c450f6a9b09ef99 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-0950000000-a65f92fe1a131e0d3933 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fs-0900000000-bc8fca766c67c27439b9 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0245998 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | 3,3'-Diaminobenzidine |
|---|
| Chemspider ID | 6804 |
|---|
| ChEBI ID | 90994 |
|---|
| PubChem Compound ID | 7071 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|