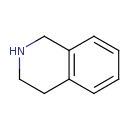| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 04:51:53 UTC |
|---|
| Update Date | 2016-11-09 01:15:13 UTC |
|---|
| Accession Number | CHEM015476 |
|---|
| Identification |
|---|
| Common Name | Isoquinoline, 1,2,3,4-tetrahydro- |
|---|
| Class | Small Molecule |
|---|
| Description | Tetrahydroisoquinoline is a secondary amine with the chemical formula C9H11N.Like other secondary amines, tetrahydroisoquinoline can be oxidized to the corresponding nitrone using hydrogen peroxide, catalyzed by selenium dioxide.The tetrahydroisoquinoline skeleton is commonly encountered in pharmaceutical drugs, notably quaternary ammonium muscle relaxants. Tetrahydroisoquinoline derivatives may be formed in the body as metabolites of some drugs, and this was once thought to be involved in the development of alcoholism.This theory has now been discredited and is no longer generally accepted by the scientific community, but endogenous production of neurotoxic tetrahydroisoquinoline derivatives such as norsalsolinol continue to be investigated as possible causes for some conditions such as Parkinson's disease.{from wiki). |
|---|
| Contaminant Sources | - FooDB Chemicals
- HPV EPA Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1234-Tetrahydroisoquinoline | ChEMBL, HMDB | | 1234-tetrahydro-Isoquinoline | ChEMBL, HMDB | | Tetrahydroisoquinoline | HMDB, MeSH | | 1,2,3,4-tetrahydro-2-Azanaphthalene | HMDB, MeSH | | 1,2,3,4-tetrahydro-2-Isoquinoline | HMDB | | 1,2,3,4-Tetrahydroleucoline | HMDB | | HCL OF 1,2,3,4-Tetrahydroisoquinoline | MeSH, HMDB | | 3,4-Dihydro-1H-isoquinoline | MeSH |
|
|---|
| Chemical Formula | C9H11N |
|---|
| Average Molecular Mass | 133.190 g/mol |
|---|
| Monoisotopic Mass | 133.089 g/mol |
|---|
| CAS Registry Number | 91-21-4 |
|---|
| IUPAC Name | 1,2,3,4-tetrahydroisoquinoline |
|---|
| Traditional Name | tetrahydroisoquinoline |
|---|
| SMILES | C1CC2=C(CN1)C=CC=C2 |
|---|
| InChI Identifier | InChI=1S/C9H11N/c1-2-4-9-7-10-6-5-8(9)3-1/h1-4,10H,5-7H2 |
|---|
| InChI Key | UWYZHKAOTLEWKK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydroisoquinolines. These are tetrahydrogenated isoquinoline derivatives. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrahydroisoquinolines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tetrahydroisoquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydroisoquinoline
- Aralkylamine
- Benzenoid
- Azacycle
- Secondary amine
- Secondary aliphatic amine
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-001i-7900000000-880ecb945a866c58a869 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-001i-7900000000-880ecb945a866c58a869 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-100r-2900000000-241c80cb6dc81b9b83e0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-9cda6a8e29a836be071a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00lr-1900000000-0fffb087a6b9e5f092fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066u-9800000000-ed1397065d659994d996 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-8b124bc9800fbbc9a3c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0900000000-7c43cb3e6b9cef43d221 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uyi-3900000000-58d4ecc958f729594d6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-1900000000-750b3fc0ed1857cd3d2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001l-4900000000-c1118a9a9962f041fb34 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9100000000-2ee9aad24737bda77796 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-163cef49a8efd2ca85d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0900000000-7cb157b4702fa57056e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ge9-2900000000-53cc67b7ef90d1c880f4 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012489 |
|---|
| FooDB ID | FDB029096 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | DIHYDRO-DIOH-BENZOATE |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Tetrahydroisoquinoline |
|---|
| Chemspider ID | 6779 |
|---|
| ChEBI ID | 114116 |
|---|
| PubChem Compound ID | 7046 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|