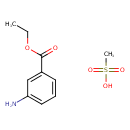
| 3-Aminobenzoic acid ethyl ester methanesulfonate | ChEBI |
| Ethyl m-aminobenzoate methane sulfonate | ChEBI |
| Ethyl m-aminobenzoate methanesulfonate | ChEBI |
| Ethyl m-aminobenzoate, methanesulfonic acid salt | ChEBI |
| Finquel | ChEBI |
| Metacaine mesylate | ChEBI |
| Metacaine methanesulfonate | ChEBI |
| MS-222 | ChEBI |
| Tricaine mesilate | ChEBI |
| Tricaine mesylate | ChEBI |
| Tricaine methane sulfonate | ChEBI |
| TS 222 | ChEBI |
| Tricaine methanesulfonate | Kegg |
| MS 222 | Kegg |
| 3-Aminobenzoate ethyl ester methanesulfonate | Generator |
| 3-Aminobenzoate ethyl ester methanesulphonate | Generator |
| 3-Aminobenzoic acid ethyl ester methanesulfonic acid | Generator |
| 3-Aminobenzoic acid ethyl ester methanesulphonic acid | Generator |
| Ethyl m-aminobenzoate methane sulphonate | Generator |
| Ethyl m-aminobenzoic acid methane sulfonic acid | Generator |
| Ethyl m-aminobenzoic acid methane sulphonic acid | Generator |
| Ethyl m-aminobenzoate methanesulphonate | Generator |
| Ethyl m-aminobenzoic acid methanesulfonic acid | Generator |
| Ethyl m-aminobenzoic acid methanesulphonic acid | Generator |
| Ethyl m-aminobenzoate, methanesulfonate salt | Generator |
| Ethyl m-aminobenzoate, methanesulphonate salt | Generator |
| Ethyl m-aminobenzoic acid, methanesulfonic acid salt | Generator |
| Ethyl m-aminobenzoic acid, methanesulphonic acid salt | Generator |
| Metacaine mesylic acid | Generator |
| Metacaine methanesulfonic acid | Generator |
| Metacaine methanesulphonate | Generator |
| Metacaine methanesulphonic acid | Generator |
| Tricaine mesilic acid | Generator |
| Tricaine mesylic acid | Generator |
| Tricaine methane sulfonic acid | Generator |
| Tricaine methane sulphonate | Generator |
| Tricaine methane sulphonic acid | Generator |
| Tricaine methanesulfonic acid | Generator |
| Tricaine methanesulphonate | Generator |
| Tricaine methanesulphonic acid | Generator |
| (3-Ethoxycarbonylphenyl)azanium;methanesulfonic acid | Generator |
| (3-Ethoxycarbonylphenyl)azanium;methanesulphonate | Generator |
| (3-Ethoxycarbonylphenyl)azanium;methanesulphonic acid | Generator |
| Metacaine | MeSH |
| Tricaine | MeSH, ChEBI |
| Tricaine methane sulfonate (CH3SO4) | MeSH |
| Tricaine hydrochloride | MeSH |
| MS222 Compound | MeSH |