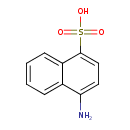
| 1-Naphthylamine-4-sulfonic acid | ChEBI |
| 4-Amino-1-naphthalene sulfonic acid | ChEBI |
| 4-Aminonaphthalin-1-sulfonsaeure | ChEBI |
| alpha-Naphthylamine-4-sulfonic acid | ChEBI |
| alpha-Naphthylamine-p-sulfonic acid | ChEBI |
| Naphthionsaeure | ChEBI |
| Piria's acid | ChEBI |
| Piria-saeure | ChEBI |
| 1-Naphthylamine-4-sulfonate | Generator |
| 1-Naphthylamine-4-sulphonate | Generator |
| 1-Naphthylamine-4-sulphonic acid | Generator |
| 4-Amino-1-naphthalene sulfonate | Generator |
| 4-Amino-1-naphthalene sulphonate | Generator |
| 4-Amino-1-naphthalene sulphonic acid | Generator |
| 4-Aminonaphthalin-1-sulphonsaeure | Generator |
| a-Naphthylamine-4-sulfonate | Generator |
| a-Naphthylamine-4-sulfonic acid | Generator |
| a-Naphthylamine-4-sulphonate | Generator |
| a-Naphthylamine-4-sulphonic acid | Generator |
| alpha-Naphthylamine-4-sulfonate | Generator |
| alpha-Naphthylamine-4-sulphonate | Generator |
| alpha-Naphthylamine-4-sulphonic acid | Generator |
| Α-naphthylamine-4-sulfonate | Generator |
| Α-naphthylamine-4-sulfonic acid | Generator |
| Α-naphthylamine-4-sulphonate | Generator |
| Α-naphthylamine-4-sulphonic acid | Generator |
| a-Naphthylamine-p-sulfonate | Generator |
| a-Naphthylamine-p-sulfonic acid | Generator |
| a-Naphthylamine-p-sulphonate | Generator |
| a-Naphthylamine-p-sulphonic acid | Generator |
| alpha-Naphthylamine-p-sulfonate | Generator |
| alpha-Naphthylamine-p-sulphonate | Generator |
| alpha-Naphthylamine-p-sulphonic acid | Generator |
| Α-naphthylamine-p-sulfonate | Generator |
| Α-naphthylamine-p-sulfonic acid | Generator |
| Α-naphthylamine-p-sulphonate | Generator |
| Α-naphthylamine-p-sulphonic acid | Generator |
| Naphthionate | Generator |
| 4-Amino-1-naphthalenesulfonic acid | MeSH |
| 4-Aminonaphthalene-1-sulfonic acid | MeSH |
| 4-Aminonaphthalenesulfonic acid | MeSH |
| Naphthionic acid, cobalt salt | MeSH |
| Naphthionic acid, copper (2+) salt (2:1) | MeSH |
| Naphthionic acid, magnesium (2:1) salt | MeSH |
| Naphthionic acid, monosodium salt | MeSH |