| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 04:46:19 UTC |
|---|
| Update Date | 2016-11-09 01:15:10 UTC |
|---|
| Accession Number | CHEM015225 |
|---|
| Identification |
|---|
| Common Name | 4-Piperidinone, 2,2,6,6-tetramethyl- |
|---|
| Class | Small Molecule |
|---|
| Description | 2,2,6,6-Tetramethyl-4-piperidinone is found in green vegetables. 2,2,6,6-Tetramethyl-4-piperidinone is isolated from Viola odorata (sweet violet |
|---|
| Contaminant Sources | - FooDB Chemicals
- HPV EPA Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
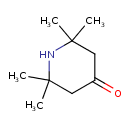
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,2,6, 6-Tetramethyl-4-piperidinone | HMDB | | 2,2,6,6-Tetramethyl-4-oxopiperidine | HMDB | | 2,2,6,6-Tetramethyl-4-piperidone | HMDB | | 2,2,6,6-Tetramethyl-g-piperidone | HMDB | | 2,2,6,6-Tetramethylpiperidin-4-one | HMDB | | 2,2,6,6-Tetramethylpiperidinone | HMDB | | 2,2,6,6-Tetramethylpiperidone | HMDB | | 2,2,6,6-Tetramethylpiperidone-4-toluene-p- sulfonate | HMDB | | 4-oxo-2,2,6,6-Tetramethyl-4-piperidone | HMDB | | 4-oxo-2,2,6,6-Tetramethylpiperidine | HMDB | | Odoratin? | HMDB | | Odoratine | HMDB | | Tempidon | HMDB | | Tmpone | HMDB | | Triacetonamin | HMDB | | Triacetonamine | HMDB | | Triacetone amine | HMDB | | Triacetoneamine | HMDB | | Trojacetonoaminy | HMDB | | Vincubina | HMDB | | Vincubine | HMDB | | Tempidon hydrochloride | HMDB | | Tempidon 4-methylbenzenesulfonate | HMDB | | Tempidon sulfate | HMDB | | 2,2,6,6-Tetramethylpiperidone-4-toluene-p-sulfonate | HMDB |
|
|---|
| Chemical Formula | C9H17NO |
|---|
| Average Molecular Mass | 155.237 g/mol |
|---|
| Monoisotopic Mass | 155.131 g/mol |
|---|
| CAS Registry Number | 826-36-8 |
|---|
| IUPAC Name | 2,2,6,6-tetramethylpiperidin-4-one |
|---|
| Traditional Name | triacetone amine |
|---|
| SMILES | CC1(C)CC(=O)CC(C)(C)N1 |
|---|
| InChI Identifier | InChI=1S/C9H17NO/c1-8(2)5-7(11)6-9(3,4)10-8/h10H,5-6H2,1-4H3 |
|---|
| InChI Key | JWUXJYZVKZKLTJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as piperidinones. Piperidinones are compounds containing a piperidine ring which bears a ketone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Piperidines |
|---|
| Sub Class | Piperidinones |
|---|
| Direct Parent | Piperidinones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Piperidinone
- Ketone
- Cyclic ketone
- Secondary aliphatic amine
- Secondary amine
- Azacycle
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0536-9300000000-7e39d8f6bb4027145493 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0536-9300000000-7e39d8f6bb4027145493 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0btd-9500000000-6054d66109b53cfd4a5b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0900000000-9a72274208611396392b | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-8737e9dbfa626f84efd2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-1900000000-07009b2dc27886e58fda | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-6900000000-2f22036288c5edcdc725 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a5c-9100000000-e6ba05d34647c96206fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-4b1d5312c8b70c0ba1f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0900000000-225d7d089b0ff1eab3f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abi-9600000000-055fd7455c182f084f91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-2dd61f17ddc1a944250e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0w29-0900000000-d39d2724ad48aafa6d21 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9200000000-5b92def7e66197390418 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9600000000-4e17935377cb9c87e971 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-bb3fd4ca35a64da356d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-ed6aa5d6c0470a07c8b6 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031179 |
|---|
| FooDB ID | FDB003198 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00058213 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 12665 |
|---|
| ChEBI ID | 292773 |
|---|
| PubChem Compound ID | 13220 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|