| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 04:37:41 UTC |
|---|
| Update Date | 2016-11-09 01:15:06 UTC |
|---|
| Accession Number | CHEM014876 |
|---|
| Identification |
|---|
| Common Name | N,N'-Diphenyl-p-benzenediamine |
|---|
| Class | Small Molecule |
|---|
| Description | An N-substituted diamine that is 1,4-phenylenediamine in which one hydrogen from each amino group is replaced by a phenyl group. |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- OECD HPV Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
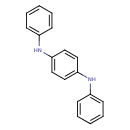
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,4-Bis(phenylamino)benzene | ChEBI | | 1,4-Dianilinobenzene | ChEBI | | 4,4'-Diphenyl-p-phenylenediamine | ChEBI | | 4-Phenylaminodiphenylamine | ChEBI | | Diphenyl-p-phenylenediamine | ChEBI | | DPPD | ChEBI | | N,N'-diphenyl-1,4-benzenediamine | ChEBI | | N,N'-diphenyl-1,4-diaminobenzene | ChEBI | | p-Bis(phenylamino)benzene | ChEBI | | p-Phenylaminodiphenylamine | ChEBI | | N,N'-diphenyl-4-phenylenediamine | HMDB | | DDPD | HMDB | | N,N'-diphenyl-1,4-phenylenediamine | HMDB | | N,N'-diphenyl-P-phenylenediamine | ChEBI |
|
|---|
| Chemical Formula | C18H16N2 |
|---|
| Average Molecular Mass | 260.340 g/mol |
|---|
| Monoisotopic Mass | 260.131 g/mol |
|---|
| CAS Registry Number | 74-31-7 |
|---|
| IUPAC Name | N1,N4-diphenylbenzene-1,4-diamine |
|---|
| Traditional Name | DFFD |
|---|
| SMILES | N(C1=CC=CC=C1)C1=CC=C(NC2=CC=CC=C2)C=C1 |
|---|
| InChI Identifier | InChI=1S/C18H16N2/c1-3-7-15(8-4-1)19-17-11-13-18(14-12-17)20-16-9-5-2-6-10-16/h1-14,19-20H |
|---|
| InChI Key | UTGQNNCQYDRXCH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aniline and substituted anilines. These are organic compounds containing an aminobenzene moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Aniline and substituted anilines |
|---|
| Direct Parent | Aniline and substituted anilines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aniline or substituted anilines
- Secondary amine
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03e9-2390000000-d040411419e49602e250 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0090000000-bd251d1ae78c2ff88fa9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03xr-1690000000-395ce6b56786da70acad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-2900000000-8f51dc6b175967c58d2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-bf39d4fa3a78097d8477 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-1090000000-944bf0b1fb52166aa039 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9330000000-9418ac04018b69f8378d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0090000000-2f16209866e7f29988de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0090000000-844d63f94b0211ee9606 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a7i-3980000000-20eed10e05bcb5308aad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-cd7631b883f8a7ce97fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0090000000-3b214a7f65745556d48f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-1390000000-c71accb47a6c4106efba | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0247345 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 6080 |
|---|
| ChEBI ID | 34860 |
|---|
| PubChem Compound ID | 6319 |
|---|
| Kegg Compound ID | C14501 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|