| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 04:33:58 UTC |
|---|
| Update Date | 2016-11-09 01:15:03 UTC |
|---|
| Accession Number | CHEM014666 |
|---|
| Identification |
|---|
| Common Name | 2-Naphthalenecarboxylic acid, 4-[(5-chloro-4-methyl-2-sulfophenyl)azo]-3-hydroxy-, calcium salt (1:1) |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
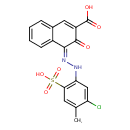
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (4Z)-4-[2-(5-Chloro-4-methyl-2-sulfophenyl)hydrazin-1-ylidene]-3-oxo-3,4-dihydronaphthalene-2-carboxylate | Generator | | (4Z)-4-[2-(5-Chloro-4-methyl-2-sulphophenyl)hydrazin-1-ylidene]-3-oxo-3,4-dihydronaphthalene-2-carboxylate | Generator | | (4Z)-4-[2-(5-Chloro-4-methyl-2-sulphophenyl)hydrazin-1-ylidene]-3-oxo-3,4-dihydronaphthalene-2-carboxylic acid | Generator |
|
|---|
| Chemical Formula | C18H13ClN2O6S |
|---|
| Average Molecular Mass | 420.820 g/mol |
|---|
| Monoisotopic Mass | 420.018 g/mol |
|---|
| CAS Registry Number | 7023-61-2 |
|---|
| IUPAC Name | (4Z)-4-[2-(5-chloro-4-methyl-2-sulfophenyl)hydrazin-1-ylidene]-3-oxo-3,4-dihydronaphthalene-2-carboxylic acid |
|---|
| Traditional Name | (4Z)-4-[2-(5-chloro-4-methyl-2-sulfophenyl)hydrazin-1-ylidene]-3-oxonaphthalene-2-carboxylic acid |
|---|
| SMILES | CC1=CC(=C(N\N=C2/C(=O)C(=CC3=CC=CC=C23)C(O)=O)C=C1Cl)S(O)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C18H13ClN2O6S/c1-9-6-15(28(25,26)27)14(8-13(9)19)20-21-16-11-5-3-2-4-10(11)7-12(17(16)22)18(23)24/h2-8,20H,1H3,(H,23,24)(H,25,26,27)/b21-16- |
|---|
| InChI Key | WAQBLNKOZKHMNC-PGMHBOJBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthalenecarboxylic acids. Naphthalenecarboxylic acids are compounds containing a naphthalene moiety, which bears a carboxylic acid group one or more positions. Naphthalene is a bicyclic compound that is made up of two fused benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthalenes |
|---|
| Sub Class | Naphthalenecarboxylic acids and derivatives |
|---|
| Direct Parent | Naphthalenecarboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-naphthalenecarboxylic acid
- Benzenesulfonate
- Benzenesulfonyl group
- 1-sulfo,2-unsubstituted aromatic compound
- Arylsulfonic acid or derivatives
- Phenylhydrazine
- Toluene
- Halobenzene
- Chlorobenzene
- Monocyclic benzene moiety
- Aryl halide
- Aryl chloride
- Sulfonyl
- Organosulfonic acid
- Organosulfonic acid or derivatives
- Organic sulfonic acid or derivatives
- Cyclic ketone
- Ketone
- Monocarboxylic acid or derivatives
- Hydrazone
- Carboxylic acid
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Carbonyl group
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-0032900000-19ca58de9fb643a85630 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1900000000-b5dd3bbf171265c68cb5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-0920000000-cd2ec44d7cce1aa6493c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0156900000-438144f49808107b0681 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00vi-1589100000-9d54aec6adc0c302874a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-1930000000-9f4da21cd463931ed8a5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6328585 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|