| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 04:18:21 UTC |
|---|
| Update Date | 2016-11-09 01:14:51 UTC |
|---|
| Accession Number | CHEM013785 |
|---|
| Identification |
|---|
| Common Name | Butanoic acid, 4-(octadecylamino)-4-oxosulfo-, ammonium salt (1:2) |
|---|
| Class | Small Molecule |
|---|
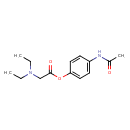
| Description | Propacetamol is a non-opioid analgesic devoid of the major contraindications.[A32051] It is a derivative of [acetaminophen], or paracetamol, with the molecular formula glycine, N, N-diethyl-,4-(acetylamino)phenyl ester. Propacetamol is a parenteral formulation of paracetamol and thus, it is a prodrug that is completely hydrolyzed to paracetamol.[A7892] It is not available in the United States but this prodrug has been widely used in other countries such as France since 1985.[L1505] |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N, N-Diethylglycine 4'-hydroxyacetanilide ester | MeSH | | Propacetamol | MeSH | | Prodafalgan | MeSH |
|
|---|
| Chemical Formula | C14H20N2O3 |
|---|
| Average Molecular Mass | 264.325 g/mol |
|---|
| Monoisotopic Mass | 264.147 g/mol |
|---|
| CAS Registry Number | 68128-59-6 |
|---|
| IUPAC Name | 4-acetamidophenyl 2-(diethylamino)acetate |
|---|
| Traditional Name | propacetamol |
|---|
| SMILES | CCN(CC)CC(=O)OC1=CC=C(NC(C)=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C14H20N2O3/c1-4-16(5-2)10-14(18)19-13-8-6-12(7-9-13)15-11(3)17/h6-9H,4-5,10H2,1-3H3,(H,15,17) |
|---|
| InChI Key | QTGAJCQTLIRCFL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alpha amino acid esters. These are ester derivatives of alpha amino acids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid ester
- Acetanilide
- Phenol ester
- N-acetylarylamine
- Anilide
- Phenoxy compound
- N-arylamide
- Monocyclic benzene moiety
- Benzenoid
- Acetamide
- Carboxamide group
- Carboxylic acid ester
- Secondary carboxylic acid amide
- Tertiary amine
- Tertiary aliphatic amine
- Monocarboxylic acid or derivatives
- Organooxygen compound
- Organonitrogen compound
- Amine
- Organic nitrogen compound
- Carbonyl group
- Organic oxide
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-9600000000-8df03cf542e3504a9ce7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01b9-2190000000-5612028d20e549ad9b5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-074r-5690000000-e6d7b90b9fbd358ba59e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a5i-9500000000-c6d596386a716de42304 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0490000000-680e71da71326fe6beb0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0mb9-3790000000-b310bb2bea82bda7ca07 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-6900000000-02cd56c181c0577c7ee8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB09288 |
|---|
| HMDB ID | HMDB0256808 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Propacetamol |
|---|
| Chemspider ID | 62097 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|