| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 04:15:14 UTC |
|---|
| Update Date | 2016-11-09 01:14:49 UTC |
|---|
| Accession Number | CHEM013601 |
|---|
| Identification |
|---|
| Common Name | Barbituric acid |
|---|
| Class | Small Molecule |
|---|
| Description | A barbiturate, the structure of which is that of perhydropyrimidine substituted at C-2, -4 and -6 by oxo groups. Barbituric acid is the parent compound of barbiturate drugs, although it is not itself pharmacologically active. |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
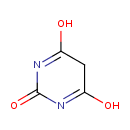
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,4,6(1H,3H,5H)-Pyrimidinetrione | ChEBI | | Barbitursaeure | ChEBI | | Malonylharnstoff | ChEBI | | Malonylurea | ChEBI | | Barbitate | Generator | | Barbitic acid | Generator | | 2,4,6(1H,3H,5H)-Pyrimidinetrione (acd/name 4.0) | HMDB | | 2,4,6-(1H,3H,5H)-Pyrimidinetrione | HMDB | | 2,4,6-Pyrimidinetriol | HMDB | | 2,4,6-Pyrimidinetrione(1H,3H,5H) | HMDB | | 2,4,6-Trihydroxypyrimidine | HMDB | | 2,4,6-Trioxohexahydropyrimidine | HMDB | | 6-Hydroxyuracil | HMDB | | Barbiturate | HMDB | | Barbitursaure | HMDB | | N,N'-(1,3-dioxo-1,3-propanediyl)-urea | HMDB | | NN'-(1,3-Dioxo-1,3-propanediyl)-urea | HMDB | | Pyrimidine-2,4,6(1H,3H,5H)-trione | HMDB | | Pyrimidinetrione | HMDB | | Barbituric acid, monosodium salt | HMDB | | Sodium barbiturate | HMDB |
|
|---|
| Chemical Formula | C4H4N2O3 |
|---|
| Average Molecular Mass | 128.086 g/mol |
|---|
| Monoisotopic Mass | 128.022 g/mol |
|---|
| CAS Registry Number | 67-52-7 |
|---|
| IUPAC Name | 4,6-dihydroxy-2,5-dihydropyrimidin-2-one |
|---|
| Traditional Name | barbituric acid |
|---|
| SMILES | OC1=NC(=O)N=C(O)C1 |
|---|
| InChI Identifier | InChI=1S/C4H4N2O3/c7-2-1-3(8)6-4(9)5-2/h1H2,(H2,5,6,7,8,9) |
|---|
| InChI Key | HNYOPLTXPVRDBG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrimidones. Pyrimidones are compounds that contain a pyrimidine ring, which bears a ketone. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Pyrimidones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidone
- Hydropyrimidine
- 2,5-dihydropyrimidine
- Carbonic acid derivative
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Azacycle
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-001j-4926000000-96492cf12ddafb9fa2c1 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-002f-9500000000-e1223bb8d4414dc44ce0 | Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-001j-4926000000-96492cf12ddafb9fa2c1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004u-9400000000-0974c3ea74bd38c34f76 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-7960000000-6fe630c5f061865ad590 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0900000000-7f6de7dbc0c8385a4986 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1900000000-488d5e5740b2d9154278 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-2900000000-c1265888f45e8b1ed0b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0059-6900000000-491964469e34c8b797d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-6bad946cb9132732634e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-b2a8b5aa5f0c3f979744 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-1900000000-cdefdcd922e108c6d3aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002o-9200000000-e88c70650db76c01b021 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-9000000000-6788917dd71900ff4a84 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004l-5900000000-69a714f826072ea5da2a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-a2a0aae677316906679f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-90726b17dc36e29c5299 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041833 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Barbituric_acid |
|---|
| Chemspider ID | 5976 |
|---|
| ChEBI ID | 16294 |
|---|
| PubChem Compound ID | 6211 |
|---|
| Kegg Compound ID | C00813 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | M2MDB005482 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|