| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 04:01:03 UTC |
|---|
| Update Date | 2016-11-09 01:14:38 UTC |
|---|
| Accession Number | CHEM012737 |
|---|
| Identification |
|---|
| Common Name | Ethanone, 1-(1,2,3,4,5,6,7,8-octahydro-2,3,8,8-tetramethyl-2-naphthalenyl)- |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
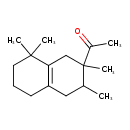
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (1,2,3,4,5,6,7,8-Octahydro-2,3,8,8-tetramethylnaphthalen-2yl)ethan-1-one | MeSH | | OHTMNE CPD | MeSH | | 1-(1,2,3,4,5,6,7,8-Octahydro-2,3,8,8-tetramethyl-2-naphthalenyl)ethanone | MeSH |
|
|---|
| Chemical Formula | C16H26O |
|---|
| Average Molecular Mass | 234.383 g/mol |
|---|
| Monoisotopic Mass | 234.198 g/mol |
|---|
| CAS Registry Number | 54464-57-2 |
|---|
| IUPAC Name | 1-(2,3,8,8-tetramethyl-1,2,3,4,5,6,7,8-octahydronaphthalen-2-yl)ethan-1-one |
|---|
| Traditional Name | 1-(2,3,8,8-tetramethyl-1,3,4,5,6,7-hexahydronaphthalen-2-yl)ethanone |
|---|
| SMILES | CC1CC2=C(CC1(C)C(C)=O)C(C)(C)CCC2 |
|---|
| InChI Identifier | InChI=1S/C16H26O/c1-11-9-13-7-6-8-15(3,4)14(13)10-16(11,5)12(2)17/h11H,6-10H2,1-5H3 |
|---|
| InChI Key | FVUGZKDGWGKCFE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as ketones. These are organic compounds in which a carbonyl group is bonded to two carbon atoms R2C=O (neither R may be a hydrogen atom). Ketones that have one or more alpha-hydrogen atoms undergo keto-enol tautomerization, the tautomer being an enol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Ketones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ketone
- Organic oxide
- Hydrocarbon derivative
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0290000000-13fdd514bc0c0f12c216 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000l-3950000000-582647b3a8a8f9e5de6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ktf-9510000000-eff0fdd17c25338ad97b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-275dbc574d1878c65931 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0290000000-c1be8e34d13ad5e11fb0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-4890000000-8a8652261f4b54985f68 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 108242 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|