| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 03:58:13 UTC |
|---|
| Update Date | 2016-11-09 01:14:36 UTC |
|---|
| Accession Number | CHEM012583 |
|---|
| Identification |
|---|
| Common Name | 1,3-Dioxane, 2,4,6-trimethyl-4-phenyl- |
|---|
| Class | Small Molecule |
|---|
| Description | 2,4,6-Trimethyl-4-phenyl-1,3-dioxane is a fragrance ingredient with a grapefruit-like arom |
|---|
| Contaminant Sources | - FooDB Chemicals
- HPV EPA Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
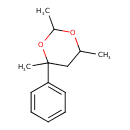
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Floralate | HMDB | | Vertacetal | HMDB |
|
|---|
| Chemical Formula | C13H18O2 |
|---|
| Average Molecular Mass | 206.281 g/mol |
|---|
| Monoisotopic Mass | 206.131 g/mol |
|---|
| CAS Registry Number | 5182-36-5 |
|---|
| IUPAC Name | 2,4,6-trimethyl-4-phenyl-1,3-dioxane |
|---|
| Traditional Name | 2,4,6-trimethyl-4-phenyl-1,3-dioxane |
|---|
| SMILES | CC1CC(C)(OC(C)O1)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C13H18O2/c1-10-9-13(3,15-11(2)14-10)12-7-5-4-6-8-12/h4-8,10-11H,9H2,1-3H3 |
|---|
| InChI Key | IEZPIUQRQRWIFE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,3-dioxanes. These are organic compounds containing 1,3-dioxane, an aliphatic six-member ring with two oxygen atoms in ring positions 1 and 3. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Dioxanes |
|---|
| Sub Class | 1,3-dioxanes |
|---|
| Direct Parent | 1,3-dioxanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzenoid
- Monocyclic benzene moiety
- Meta-dioxane
- Oxacycle
- Acetal
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0i04-1900000000-0d538799d56a9525803d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-1970000000-d5dec8c812c4fec52d03 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ow-1900000000-935217a2ecd9b9bc0cc5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-5900000000-ae5b769b6aaaa32be922 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-2290000000-3466e457b5a45de22c98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0bt9-9530000000-4405d0fdbe906728db1c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00mo-9800000000-7ea9dcf0d0c8681994f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-3390000000-ab2bed6819d93f9a054f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-9610000000-5e5b4c273705633988e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-9400000000-fe6e33c4ecf694713a04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0950000000-5c8a0083ca507090c888 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ar0-3900000000-9bb51e111a494fd40232 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056u-6900000000-78504cdd81613a5beac8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036025 |
|---|
| FooDB ID | FDB014843 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 96644 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 107381 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|