| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 03:56:55 UTC |
|---|
| Update Date | 2016-11-09 01:14:35 UTC |
|---|
| Accession Number | CHEM012499 |
|---|
| Identification |
|---|
| Common Name | 1H-Pyrazole-5-carboxamide, 3-bromo-N-[4-chloro-2-methyl-6-[(methylamino)carbonyl]phenyl]-1-(3-chloro-2-pyridinyl)- |
|---|
| Class | Small Molecule |
|---|
| Description | A carboxamide resulting from the formal condensation of the carboxylic acid group of 3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid with the primary amino group of 2-amino-5-chloro-N,3-dimethylbenzamide. The first of the anthranilic diamide insecticides, it is a ryanodine receptor activator and is used to protect a wide variety of crops, including corn, cotton, grapes, rice and potatoes. |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
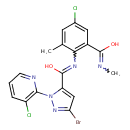
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Bromo-N-{4-chloro-2-methyl-6-[(methylamino)carbonyl]phenyl}-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxamide | ChEBI | | DPX-e2Y45 | ChEBI | | Rynaxpyr | ChEBI | | Chlorantranilipole | MeSH | | 3-Bromo-N-(4-chloro-2-methyl-6-((methylamino)carbonyl)phenyl)-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxamide | MeSH | | Rynaxypyr | MeSH | | 3-Bromo-N-[4-chloro-2-methyl-6-(methyl-C-hydroxycarbonimidoyl)phenyl]-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboximidate | Generator |
|
|---|
| Chemical Formula | C18H14BrCl2N5O2 |
|---|
| Average Molecular Mass | 483.150 g/mol |
|---|
| Monoisotopic Mass | 480.971 g/mol |
|---|
| CAS Registry Number | 500008-45-7 |
|---|
| IUPAC Name | 3-bromo-N-[4-chloro-2-methyl-6-(methyl-C-hydroxycarbonimidoyl)phenyl]-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboximidic acid |
|---|
| Traditional Name | 5-bromo-N-[4-chloro-2-methyl-6-(methyl-C-hydroxycarbonimidoyl)phenyl]-2-(3-chloropyridin-2-yl)pyrazole-3-carboximidic acid |
|---|
| SMILES | CN=C(O)C1=C(N=C(O)C2=CC(Br)=NN2C2=C(Cl)C=CC=N2)C(C)=CC(Cl)=C1 |
|---|
| InChI Identifier | InChI=1S/C18H14BrCl2N5O2/c1-9-6-10(20)7-11(17(27)22-2)15(9)24-18(28)13-8-14(19)25-26(13)16-12(21)4-3-5-23-16/h3-8H,1-2H3,(H,22,27)(H,24,28) |
|---|
| InChI Key | PSOVNZZNOMJUBI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrazolylpyridines. Pyrazolylpyridines are compounds containing a pyrazolylpyridine skeleton, which consists of a pyrazole linked (not fused) to a pyridine by a bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyrazolylpyridines |
|---|
| Direct Parent | Pyrazolylpyridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-pyrazolylpyridine
- Chlorobenzene
- Halobenzene
- Toluene
- Aryl bromide
- Aryl chloride
- Aryl halide
- Monocyclic benzene moiety
- Benzenoid
- Azole
- Heteroaromatic compound
- Pyrazole
- Azacycle
- Carboximidic acid
- Carboximidic acid derivative
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organohalogen compound
- Organobromide
- Organochloride
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - NA , positive | splash10-0f89-0590400000-f730d8a156e6dacad7b1 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - NA , positive | splash10-0f89-0590400000-40aaba7719f54cad75aa | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - NA , positive | splash10-0udi-0000090000-724c7516ca5799611f0d | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - NA , negative | splash10-0ufu-5490000000-886bc259ce024af6c3f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001j-0500900000-c3f4544b32eb2ec66fce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00l2-0900100000-4b3ce290c1260b9322db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0900000000-5e274f50ed7f1fad4fbf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0010900000-286e65015f6796c46022 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udj-0323900000-c7292b5a0374cf645fd9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-3900000000-951cc3ab31485e71865b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Chlorantraniliprole |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 67113 |
|---|
| PubChem Compound ID | 11271640 |
|---|
| Kegg Compound ID | C18454 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|