| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 03:56:29 UTC |
|---|
| Update Date | 2016-11-09 01:14:35 UTC |
|---|
| Accession Number | CHEM012478 |
|---|
| Identification |
|---|
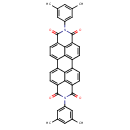
| Common Name | Anthra[2,1,9-def:6,5,10-d'e'f']diisoquinoline-1,3,8,10(2H,9H)-tetrone, 2,9-bis(3,5-dimethylphenyl)- |
|---|
| Class | Small Molecule |
|---|
| Description | FDA approved colourant for food contact polymers |
|---|
| Contaminant Sources | - FooDB Chemicals
- HPV EPA Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C40H26N2O4 |
|---|
| Average Molecular Mass | 598.645 g/mol |
|---|
| Monoisotopic Mass | 598.189 g/mol |
|---|
| CAS Registry Number | 4948-15-6 |
|---|
| IUPAC Name | 7,18-bis(3,5-dimethylphenyl)-7,18-diazaheptacyclo[14.6.2.2²,⁵.0³,¹².0⁴,⁹.0¹³,²³.0²⁰,²⁴]hexacosa-1(23),2,4,9,11,13,15,20(24),21,25-decaene-6,8,17,19-tetrone |
|---|
| Traditional Name | 7,18-bis(3,5-dimethylphenyl)-7,18-diazaheptacyclo[14.6.2.2²,⁵.0³,¹².0⁴,⁹.0¹³,²³.0²⁰,²⁴]hexacosa-1(23),2,4,9,11,13,15,20(24),21,25-decaene-6,8,17,19-tetrone |
|---|
| SMILES | CC1=CC(=CC(C)=C1)N1C(=O)C2=CC=C3C4=CC=C5C(=O)N(C6=CC(C)=CC(C)=C6)C(=O)C6=C5C4=C(C=C6)C4=C3C2=C(C=C4)C1=O |
|---|
| InChI Identifier | InChI=1S/C40H26N2O4/c1-19-13-20(2)16-23(15-19)41-37(43)29-9-5-25-27-7-11-31-36-32(40(46)42(39(31)45)24-17-21(3)14-22(4)18-24)12-8-28(34(27)36)26-6-10-30(38(41)44)35(29)33(25)26/h5-18H,1-4H3 |
|---|
| InChI Key | FDXVHZCFTCIKDD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenanthrenes and derivatives. These are polycyclic compounds containing a phenanthrene moiety, which is a tricyclic aromatic compound with three non-linearly fused benzene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenanthrenes and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Phenanthrenes and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Anthracene
- Phenanthrene
- Isoquinolone
- Isoquinoline
- M-xylene
- Xylene
- Pyridinone
- Pyridine
- Monocyclic benzene moiety
- Heteroaromatic compound
- Lactam
- Azacycle
- Organoheterocyclic compound
- Organic nitrogen compound
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-0300390000-438766a685722ed74c1e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000090000-31a3e8d56df86c83fe7a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0000090000-d6d24006d48ce7d3c6ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00l2-1000090000-b920ceedb98087f8bffe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000090000-d39376bb36c2b94d2226 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0000090000-9336ac55a26784a8493b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0000090000-bf6ac0091b4abc43880e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000090000-cf56406c8316d7041e91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0000090000-cf56406c8316d7041e91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0005-0000190000-5aeafe0c7514c1070eba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000090000-0f8545fe0f2ba8d0240a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0000090000-0f8545fe0f2ba8d0240a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0000190000-7581fd284ace7136c40c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031986 |
|---|
| FooDB ID | FDB008679 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 56322 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 62555 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|