| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 03:43:58 UTC |
|---|
| Update Date | 2016-11-09 01:14:26 UTC |
|---|
| Accession Number | CHEM011735 |
|---|
| Identification |
|---|
| Common Name | Quino[2,3-b]acridine-7,14-dione, 4,11-dichloro-5,12-dihydro- |
|---|
| Class | Small Molecule |
|---|
| Description | 4,11-Dichloro-5,12-dihydroquino[2,3-b]acridine-7,14-dione is a fda approved colourant for food contact polymers. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HPV EPA Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
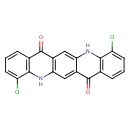
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4,11 DCQA | HMDB | | 4,11-dichloro Quinacridone | HMDB | | 4,11-dichloro-5,12-dihydroquino(2,3-b)Acridine-7,14-dione | HMDB | | 4,11-dichloro-Quinacridone | HMDB |
|
|---|
| Chemical Formula | C20H10Cl2N2O2 |
|---|
| Average Molecular Mass | 381.212 g/mol |
|---|
| Monoisotopic Mass | 380.012 g/mol |
|---|
| CAS Registry Number | 3089-16-5 |
|---|
| IUPAC Name | 4,11-dichloro-5,7,12,14-tetrahydro-5,12-diazapentacene-7,14-dione |
|---|
| Traditional Name | 4,11-dichloro-5,12-dihydro-5,12-diazapentacene-7,14-dione |
|---|
| SMILES | ClC1=CC=CC2=C1NC1=CC3=C(NC4=C(C=CC=C4Cl)C3=O)C=C1C2=O |
|---|
| InChI Identifier | InChI=1S/C20H10Cl2N2O2/c21-13-5-1-3-9-17(13)23-15-8-12-16(7-11(15)19(9)25)24-18-10(20(12)26)4-2-6-14(18)22/h1-8H,(H,23,25)(H,24,26) |
|---|
| InChI Key | BFEJTCHFLJECJN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyridoacridines. Pyridoacridines are compounds containing a pyridoacridine ring system, which consists of a pyridine fused to an acridine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Benzoquinolines |
|---|
| Direct Parent | Pyridoacridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridoacridine
- Acridone
- Dihydroquinolone
- Chloroquinoline
- Haloquinoline
- Dihydroquinoline
- Aryl chloride
- Aryl halide
- Pyridine
- Benzenoid
- Heteroaromatic compound
- Vinylogous amide
- Azacycle
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-0119000000-4c1e57144d0d98652743 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-b379c57700bea1fc1b37 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0009000000-ed271c4314aeb25d0d75 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0019000000-49d368f51e42cb650cfc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-1344d8109aa74ac31907 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0009000000-1df48f64c2acfe0b3e91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fbc-0029000000-924820a3f1f59960b0c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-2647694bfe524089479c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-005c-4009000000-f7706321a0c45d466d39 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9004000000-800e4ab54c4456cbc1de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-3c00f8283cdcd1688bb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0009000000-3c00f8283cdcd1688bb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udj-0019000000-079655b4e10efd314621 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037810 |
|---|
| FooDB ID | FDB016955 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 68998 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 76529 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|