| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 03:39:03 UTC |
|---|
| Update Date | 2016-11-09 01:14:23 UTC |
|---|
| Accession Number | CHEM011457 |
|---|
| Identification |
|---|
| Common Name | Phenol, 2-[4,6-bis(2,4-dimethylphenyl)-1,3,5-triazin-2-yl]-5-(octyloxy)- |
|---|
| Class | Small Molecule |
|---|
| Description | FDA permitted light stabiliser for food-contact olefinic polymers. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HPV EPA Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
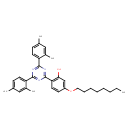
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-[4,6-Di-(2,4-xylyl)-S-triazin-2-yl]-5-(octyloxy)phenol, 8ci | HMDB | | Cysorb uv-1164 | HMDB |
|
|---|
| Chemical Formula | C33H39N3O2 |
|---|
| Average Molecular Mass | 509.682 g/mol |
|---|
| Monoisotopic Mass | 509.304 g/mol |
|---|
| CAS Registry Number | 2725-22-6 |
|---|
| IUPAC Name | 2-[bis(2,4-dimethylphenyl)-1,3,5-triazin-2-yl]-5-(octyloxy)phenol |
|---|
| Traditional Name | 2-[bis(2,4-dimethylphenyl)-1,3,5-triazin-2-yl]-5-(octyloxy)phenol |
|---|
| SMILES | CCCCCCCCOC1=CC(O)=C(C=C1)C1=NC(=NC(=N1)C1=CC=C(C)C=C1C)C1=CC=C(C)C=C1C |
|---|
| InChI Identifier | InChI=1S/C33H39N3O2/c1-6-7-8-9-10-11-18-38-26-14-17-29(30(37)21-26)33-35-31(27-15-12-22(2)19-24(27)4)34-32(36-33)28-16-13-23(3)20-25(28)5/h12-17,19-21,37H,6-11,18H2,1-5H3 |
|---|
| InChI Key | ZSSVCEUEVMALRD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenol ethers. These are aromatic compounds containing an ether group substituted with a benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenol ethers |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Phenol ethers |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenoxy compound
- M-xylene
- Xylene
- Phenol ether
- Alkyl aryl ether
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- 1,3,5-triazine
- Triazine
- Heteroaromatic compound
- Ether
- Azacycle
- Organoheterocyclic compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002n-9002200000-5deb94c0fda18851c720 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0zmi-9000530000-65640ebe5921e13de41f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-1201190000-cc1ac35b874f19dfd3cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-9807450000-207cb1b3abe69d293eb1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0536-9103000000-6765f421461f230ff063 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0004190000-3808946c44f723419e68 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009230000-013681c2d98e704f0755 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fba-0009000000-79222ce160e22bb6cf3a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000090000-b0b750124a44577992da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-5003960000-14c9fd4993e1b8f88f93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001r-2009200000-b393d2af09586eded00c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4j-0006090000-9f5b3e277d0a91b943e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0004490000-a6794991a23978751ba0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00pj-0129100000-061fd43b4ad169d1a5da | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037802 |
|---|
| FooDB ID | FDB016947 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10632726 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 135414248 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|