| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 03:39:01 UTC |
|---|
| Update Date | 2016-11-09 01:14:23 UTC |
|---|
| Accession Number | CHEM011455 |
|---|
| Identification |
|---|
| Common Name | 1,2-Benzenedicarboxamide, N2-[1,1-dimethyl-2-(methylsulfonyl)ethyl]-3-iodo-N1-[2-methyl-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]phenyl]- |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
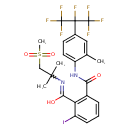
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N(2)-[1,1-Dimethyl-2-(methylsulfonyl)ethyl]-3-iodo-N(1)-{2-methyl-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]phenyl}phthalamide | ChEBI | | N(2)-[1,1-Dimethyl-2-(methylsulphonyl)ethyl]-3-iodo-N(1)-{2-methyl-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]phenyl}phthalamide | Generator | | 1-N-[4-(1,1,1,2,3,3,3-Heptafluoropropan-2-yl)-2-methylphenyl]-3-iodo-2-N-(2-methyl-1-methylsulphonylpropan-2-yl)benzene-1,2-dicarboxamide | Generator | | Flubendiamide | MeSH | | 3-iodo-N-(2-Methanesulfonyl-1,1-dmethylethyl)-n'-(2-methyl-4-(1,2,2,2-tetrafluoro-1-trifluoromethylethyl)phenyl)phthalamide | MeSH |
|
|---|
| Chemical Formula | C23H22F7IN2O4S |
|---|
| Average Molecular Mass | 682.390 g/mol |
|---|
| Monoisotopic Mass | 682.023 g/mol |
|---|
| CAS Registry Number | 272451-65-7 |
|---|
| IUPAC Name | 2-{[4-(1,1,1,2,3,3,3-heptafluoropropan-2-yl)-2-methylphenyl]carbamoyl}-6-iodo-N-(1-methanesulfonyl-2-methylpropan-2-yl)benzene-1-carboximidic acid |
|---|
| Traditional Name | 2-{[4-(1,1,1,2,3,3,3-heptafluoropropan-2-yl)-2-methylphenyl]carbamoyl}-6-iodo-N-(1-methanesulfonyl-2-methylpropan-2-yl)benzenecarboximidic acid |
|---|
| SMILES | CC1=CC(=CC=C1NC(=O)C1=C(C(O)=NC(C)(C)CS(C)(=O)=O)C(I)=CC=C1)C(F)(C(F)(F)F)C(F)(F)F |
|---|
| InChI Identifier | InChI=1S/C23H22F7IN2O4S/c1-12-10-13(21(24,22(25,26)27)23(28,29)30)8-9-16(12)32-18(34)14-6-5-7-15(31)17(14)19(35)33-20(2,3)11-38(4,36)37/h5-10H,11H2,1-4H3,(H,32,34)(H,33,35) |
|---|
| InChI Key | ZGNITFSDLCMLGI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzanilides. These are aromatic compounds containing an anilide group in which the carboxamide group is substituted with a benzene ring. They have the general structure RNC(=O)R', where R,R'= benzene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Anilides |
|---|
| Direct Parent | Benzanilides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzanilide
- Halobenzoic acid or derivatives
- 3-halobenzoic acid or derivatives
- 2-halobenzoic acid or derivatives
- Benzamide
- Benzoic acid or derivatives
- Benzoyl
- Toluene
- Halobenzene
- Iodobenzene
- Aryl halide
- Aryl iodide
- Vinylogous halide
- Sulfonyl
- Sulfone
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid derivative
- Organoiodide
- Organofluoride
- Organohalogen compound
- Organosulfur compound
- Alkyl halide
- Alkyl fluoride
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-004l-1900000000-36476f299db3383aa226 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Negative | splash10-00b9-2900000000-8aba1f27e62f3e3a349b | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Negative | splash10-004l-1900000000-23cae6d0d061cc82b171 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-0udi-0090000000-43e79c4d6cd5ce6a2d28 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-0udi-0090000000-4e5345ca8985c65ae89c | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Negative | splash10-002f-0920000000-6de5f80d8083d1e6fb3e | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-0w29-0290000000-cfed0b51b0b8a04273d9 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-0w29-0290000000-5a0f7e35be657f7d73ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000029000-f403a64c2dd61775e2ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f80-0010094000-d74c843a21a0d2d56086 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ugr-1141090000-81dc48dfbf4f4f904bea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-017i-5900007000-d7e9b371994fd7924309 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9201024000-cc18ec8c27c4c9b79595 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fb9-9312010000-03399af497926165561c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000049000-c87cbb489305dca1166a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0kcs-1152295000-e6da60f443defb9b00e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ai-1091030000-aa9ea3d9e1fedc93df8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0000009000-570a932a4b26b3a3fa8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-7421094000-208ba4610bb98aba9cc0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-4982110000-c8cdf55036fa245e1cc3 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Flubendiamide |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 38798 |
|---|
| PubChem Compound ID | 11193251 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|