| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 03:31:23 UTC |
|---|
| Update Date | 2016-11-09 01:14:18 UTC |
|---|
| Accession Number | CHEM011004 |
|---|
| Identification |
|---|
| Common Name | 1,3-Benzenedicarboxylic acid, 5-sulfo- |
|---|
| Class | Small Molecule |
|---|
| Description | 5-Sulfo-1,3-benzenedicarboxylic acid is a fda approved for use in food-contact polyester resins. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HPV EPA Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
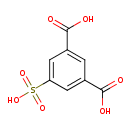
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-SulfO-1,3-benzenedicarboxylate | Generator | | 5-SulphO-1,3-benzenedicarboxylate | Generator | | 5-SulphO-1,3-benzenedicarboxylic acid | Generator | | 5-SulfO-isophthalic acid | ChEMBL, HMDB | | 5-SulfO-isophthalate | Generator, HMDB | | 5-SulphO-isophthalate | Generator, HMDB | | 5-SulphO-isophthalic acid | Generator, HMDB | | 1,3-Benzenedicarboxylic acid, 5-sulfO-, monopotassium salt | HMDB | | 5-SulfO-1,3-benzenedicarboxylic acid, monopotassium salt | HMDB | | 5-Sulfoisophthalic acid | HMDB | | 5-Sulfoisophthalic acid, 8ci | HMDB | | 5-Sulphoisophthalic acid | HMDB | | Isophthalic acid, 5-sulfO- (6ci,7ci,8ci) | HMDB | | Monopotassium 5-sulfoisophthalate | HMDB | | 5-Sulfobenzene-1,3-dicarboxylate | Generator | | 5-Sulphobenzene-1,3-dicarboxylate | Generator | | 5-Sulphobenzene-1,3-dicarboxylic acid | Generator |
|
|---|
| Chemical Formula | C8H6O7S |
|---|
| Average Molecular Mass | 246.194 g/mol |
|---|
| Monoisotopic Mass | 245.983 g/mol |
|---|
| CAS Registry Number | 22326-31-4 |
|---|
| IUPAC Name | 5-sulfobenzene-1,3-dicarboxylic acid |
|---|
| Traditional Name | 5-sulfobenzene-1,3-dicarboxylic acid |
|---|
| SMILES | OC(=O)C1=CC(=CC(=C1)C(O)=O)S(O)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C8H6O7S/c9-7(10)4-1-5(8(11)12)3-6(2-4)16(13,14)15/h1-3H,(H,9,10)(H,11,12)(H,13,14,15) |
|---|
| InChI Key | CARJPEPCULYFFP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 3-sulfobenzoic acids. These are sulfobenzoic acid carrying the sulfonyl group at the 3-position of the benzene group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzenesulfonic acids and derivatives |
|---|
| Direct Parent | 3-sulfobenzoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-sulfobenzoic acid
- Meta_phthalic_acid
- Arylsulfonic acid or derivatives
- Benzenesulfonyl group
- Benzoic acid or derivatives
- 1-sulfo,2-unsubstituted aromatic compound
- Benzoic acid
- Benzoyl
- Dicarboxylic acid or derivatives
- Sulfonyl
- Organosulfonic acid
- Organosulfonic acid or derivatives
- Organic sulfonic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organosulfur compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fba-2890000000-068e2266d00dbc77ec87 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0fk9-7029000000-837f3df38c093311a68a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-8ecea0c176e1cf9e4282 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0190000000-4b0af861aad76dc3e475 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00xs-3920000000-baa50dd6e62e3cb2ad64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-b59c451382c42e56e422 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udl-0390000000-5b6404d6423ae1d3472d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fr-1920000000-81dcb60c6d48a194dfe6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-a0c731a407be2183881c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0090000000-427ea3e6610a3cf5215d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9620000000-6d9e346ab42f458ac518 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-2887b71060c630b83e1b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0090000000-5ccf82881e6a48af7207 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uk9-0940000000-96eeeeb34a159b066545 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032822 |
|---|
| FooDB ID | FDB010798 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 72881 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 80714 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|