| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 03:21:31 UTC |
|---|
| Update Date | 2016-11-09 01:14:11 UTC |
|---|
| Accession Number | CHEM010382 |
|---|
| Identification |
|---|
| Common Name | Advastab 17 mok |
|---|
| Class | Small Molecule |
|---|
| Description | Dioctyltin isooctylthioglycolate is used as a heat stabiliser for rigid PVC used in food and drink application |
|---|
| Contaminant Sources | - FooDB Chemicals
- HPV EPA Chemicals
- OECD HPV Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
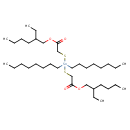
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Dioctyltin isooctylthioglycolic acid | Generator | | 2-Ethylhexyl 10-ethyl-4,4-dioctyl-7-oxo-8-oxa-3,5-dithia-4-stannatetradecanoate, 14ci | HMDB | | Advastab 17 mok | HMDB | | Advastab 17mol | HMDB | | Bis(2-ethylhexyl thioglycolato)dioctyltin | HMDB | | Bis(2-ethylhexyl) ((dioctylstannylene)dithio)diacetate | HMDB | | Di-(N-octyl)tin S,s'-bis(isooctylmercaptoacetate) | HMDB | | Di-N-octyltin bis(2-ethylhexyl mercaptoacetate) | HMDB | | Di-N-octyltin-2-ethylhexyl-dimercaptoethanoate | HMDB | | Di-N-octyltin-dithioglycolic acid 2-ethylhexyl ester | HMDB | | Dioctyltin bis(2-ethylhexyl thioglycolate) | HMDB | | Dioctyltin bis(2-ethylhexylmercaptoacetate) | HMDB | | Dioctyltin bis(isooctylmercaptoacetate) | HMDB | | Dioctyltin S,s'-bis(2-ethylhexylthioglycolate) | HMDB | | Dioctyltinbis(2-ethylhexyl mercaptoacetate) | HMDB | | Tin, bis(mercaptoacetate)dioctyl-, bis(2-ethylhexyl) ester | HMDB | | Tin, dioctyl-, bis(2-ethylhexylthioglycolate) | HMDB | | Irgastab 17 mok | HMDB | | Di-N-octyltin-2-ethyl-N-hexyldithioglycollate | HMDB |
|
|---|
| Chemical Formula | C36H72O4S2Sn |
|---|
| Average Molecular Mass | 751.790 g/mol |
|---|
| Monoisotopic Mass | 752.389 g/mol |
|---|
| CAS Registry Number | 15571-58-1 |
|---|
| IUPAC Name | 2-ethylhexyl 2-{[({2-[(2-ethylhexyl)oxy]-2-oxoethyl}sulfanyl)dioctylstannyl]sulfanyl}acetate |
|---|
| Traditional Name | advastab 17 mok |
|---|
| SMILES | CCCCCCCC[Sn](CCCCCCCC)(SCC(=O)OCC(CC)CCCC)SCC(=O)OCC(CC)CCCC |
|---|
| InChI Identifier | InChI=1S/2C10H20O2S.2C8H17.Sn/c2*1-3-5-6-9(4-2)7-12-10(11)8-13;2*1-3-5-7-8-6-4-2;/h2*9,13H,3-8H2,1-2H3;2*1,3-8H2,2H3;/q;;;;+2/p-2 |
|---|
| InChI Key | VXGIVDFKZKMKQO-UHFFFAOYSA-L |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dicarboxylic acids and derivatives. These are organic compounds containing exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Dicarboxylic acids and derivatives |
|---|
| Direct Parent | Dicarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dicarboxylic acid or derivatives
- Carboxylic acid ester
- Dialkyltin salt
- Sulfenyl compound
- Organic metal salt
- Dialkyltin
- Carbonyl group
- Organic salt
- Organosulfur compound
- Organooxygen compound
- Organometallic compound
- Organic post-transition metal moeity
- Organic oxide
- Organic tin salt
- Organic oxygen compound
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-5910022000-b0ee892ca2b6c7a69e68 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000000900-129f983a714577251046 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0000000900-129f983a714577251046 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0000000900-129f983a714577251046 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000000900-ec3fa416b91ab37c466a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0000000900-ec3fa416b91ab37c466a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0000000900-ec3fa416b91ab37c466a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udj-0000041900-450b073a47d567b2d391 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052b-9300041000-5478f8695a0220a807fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0900-9700000000-c5f6d88b8a853fe6b740 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000040900-63382b63f1b58bf0cff7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0208290100-6afde1847263bcb57dda | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-2400629100-b7a74cd75ce793596e25 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031615 |
|---|
| FooDB ID | FDB008252 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 7850483 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 15978303 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|