| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 03:20:14 UTC |
|---|
| Update Date | 2016-11-09 01:14:10 UTC |
|---|
| Accession Number | CHEM010300 |
|---|
| Identification |
|---|
| Common Name | Tiron |
|---|
| Class | Small Molecule |
|---|
| Description | Chymopapain is found in fruits. Chymopapain is isolated from Carica papaya (papaya |
|---|
| Contaminant Sources | - FooDB Chemicals
- HPV EPA Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
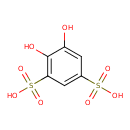
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2-Dihydroxybenzene-3,5-disulfonic acid disodium salt | HMDB | | 1,2-Dihydroxybenzene-3,5-disulfonate disodium salt | HMDB | | 1,2-Dihydroxybenzene-3,5-disulphonate disodium salt | HMDB | | 1,2-Dihydroxybenzene-3,5-disulphonic acid disodium salt | HMDB | | 1,2-DIHYDROXYBENZENE-3,5-disulfonIC ACID,di na salt | HMDB | | 1,3-Benzenedisulfonic acid, 4,5-dihydroxy-, disodium salt | HMDB | | 149-46-2 (Parent CPD) | HMDB | | 3,5-Disulfocatechol disodium salt | HMDB | | 4,5-DIHYDROXY-1,3-benzenedisulfonIC ACID | HMDB | | 4,5-Dihydroxy-1,3-benzenedisulfonic acid disodium salt | HMDB | | 4,5-Dihydroxy-m-benzenedisulfonic acid disodium salt | HMDB | | BAX 1526 | HMDB | | Chymodiactin | HMDB | | Dihydroxy benzene disulfonate disodium salt | HMDB | | Discase | HMDB | | Disodium 1,2-dihydroxybenzene-3,5-disulfonate | HMDB | | Disodium 4,5-dihydroxy-1,3-benzenedisulfonate | HMDB | | Disodium 4,5-dihydroxy-m-benzenedisulfonate | HMDB | | Disodium 4,5-dihydroxybenzene-1,3-disulfonate | HMDB | | Disodium 4,5-dihydroxybenzene-1,3-disulphonate | HMDB | | Disodium pyrocatechol-3,5-disulfonate | HMDB | | m-Benzenedisulfonic acid, 4,5-dihydroxy-, disodium salt | HMDB | | NSC 107079 | HMDB | | Pyrocatechol-3,5-disulfonic acid disodium salt | HMDB | | SDD | HMDB | | Sodium 1,2-dihydroxy-3,5-benzenedisulfonate | HMDB | | Sodium 1,2-dihydroxybenzenedisulfonate | HMDB | | Sodium 4,5-dihydroxybenzene-1,3-disulfonate | HMDB | | Sodium catechol sulfate | HMDB | | Sodium catechol sulphate | HMDB | | Sodium pyrocatechol-3,5-disulfonate | HMDB | | Tiferron | HMDB | | Tiron | HMDB | | Tiron(R) | HMDB | | 4,5-Dihydroxybenzene-1,3-disulfonate | HMDB | | 4,5-Dihydroxybenzene-1,3-disulphonate | HMDB | | 4,5-Dihydroxybenzene-1,3-disulphonic acid | HMDB | | Chemolase | HMDB | | Chymopapain a | HMDB | | Chymopapain b | HMDB | | Sodium-4,5-dihydroxy-1,3-benzene disulfonate | HMDB |
|
|---|
| Chemical Formula | C6H4Na2O8S2 |
|---|
| Average Molecular Mass | 314.201 g/mol |
|---|
| Monoisotopic Mass | 313.914 g/mol |
|---|
| CAS Registry Number | 149-45-1 |
|---|
| IUPAC Name | 4,5-dihydroxybenzene-1,3-disulfonic acid |
|---|
| Traditional Name | tiron |
|---|
| SMILES | [Na+].[Na+].OC1=CC(=CC(=C1O)S([O-])(=O)=O)S([O-])(=O)=O |
|---|
| InChI Identifier | InChI=1S/C6H6O8S2.2Na/c7-4-1-3(15(9,10)11)2-5(6(4)8)16(12,13)14;;/h1-2,7-8H,(H,9,10,11)(H,12,13,14);;/q;2*+1/p-2 |
|---|
| InChI Key | ISWQCIVKKSOKNN-UHFFFAOYSA-L |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzenesulfonic acids and derivatives. These are organic compounds containing a sulfonic acid or a derivative thereof that is linked to a benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzenesulfonic acids and derivatives |
|---|
| Direct Parent | Benzenesulfonic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzenesulfonate
- Arylsulfonic acid or derivatives
- Benzenesulfonyl group
- 1-sulfo,2-unsubstituted aromatic compound
- Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Organosulfonic acid
- Sulfonyl
- Hydrocarbon derivative
- Organosulfur compound
- Organic oxygen compound
- Organic oxide
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-ee475594b730b56c6eb0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0940000000-1e5750f1431e6cbd6c77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-1900000000-d4f4308734952b0dd39c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-2090000000-a531d791554411d3d07d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-015i-5590000000-e77e665626e6331157df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9300000000-604f74c133b0dc6e1764 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fk9-0090000000-4079438743e1c69aa57c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0190000000-bb4932b8faa638c46cb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-9400000000-ecb405f2be85fcc885ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-c1230a2d7270d7a59246 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-1090000000-2c961ab3fb0ca78594b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9700000000-a6d03b28abe7fcf973d1 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029845 |
|---|
| FooDB ID | FDB031408 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Chymopapain |
|---|
| Chemspider ID | 8652 |
|---|
| ChEBI ID | 278533 |
|---|
| PubChem Compound ID | 9002 |
|---|
| Kegg Compound ID | C11159 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Porter RW, Ralston SH: Pharmacological management of back pain syndromes. Drugs. 1994 Aug;48(2):189-98. | | 2. Sawin PD, Traynelis VC, Rich G, Smith BA, Maves TJ, Follett KA, Moore SA: Chymopapain-induced reduction of proinflammatory phospholipase A2 activity and amelioration of neuropathic behavioral changes in an in vivo model of acute sciatica. J Neurosurg. 1997 Jun;86(6):998-1006. | | 3. Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC. |
|
|---|