| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 03:18:04 UTC |
|---|
| Update Date | 2016-11-09 01:14:09 UTC |
|---|
| Accession Number | CHEM010184 |
|---|
| Identification |
|---|
| Common Name | 2-Aminoethane dodecanoate |
|---|
| Class | Small Molecule |
|---|
| Description | An N-(long-chain-acyl)ethanolamine resulting from the formal condensation of the carboxy group of dodecanoic acid (myristic acid) with the amino group of ethanolamine. |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
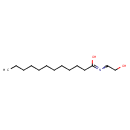
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Amisol lme | ChEBI | | Comperlan LM | ChEBI | | Copramyl | ChEBI | | Crillon lme | ChEBI | | Cyclomide LM | ChEBI | | Dodecanoyl ethanolamide | ChEBI | | Lauramide mea | ChEBI | | Lauric acid ethanolamide | ChEBI | | Lauric acid monoethanolamide | ChEBI | | Lauric acid monoethanolamine | ChEBI | | Lauric ethylolamide | ChEBI | | Lauric monoethanolamide | ChEBI | | Lauridit LM | ChEBI | | Lauroyl monoethanolamide | ChEBI | | Lauroyl-ea | ChEBI | | Lauroyl-ethanolamine | ChEBI | | Monoethanolamine lauric acid amide | ChEBI | | N-(2-Hydroxyethyl)lauramide | ChEBI | | N-(Dodecanoyl)-ethanolamine | ChEBI | | N-Dodecanoylethanolamine | ChEBI | | N-Lauroylethanolamine | ChEBI | | Rewomid L 203 | ChEBI | | Rolamid CM | ChEBI | | Stabilor CMH | ChEBI | | Steinamid L 203 | ChEBI | | Ultrapole H | ChEBI | | Vistalan | ChEBI | | Laate ethanolamide | Generator | | Laic acid ethanolamide | Generator | | Laate monoethanolamide | Generator | | Laic acid monoethanolamide | Generator | | Laate monoethanolamine | Generator | | Laic acid monoethanolamine | Generator | | Monoethanolamine laate amide | Generator | | Monoethanolamine laic acid amide | Generator | | N-LEA | MeSH |
|
|---|
| Chemical Formula | C14H29NO2 |
|---|
| Average Molecular Mass | 243.391 g/mol |
|---|
| Monoisotopic Mass | 243.220 g/mol |
|---|
| CAS Registry Number | 142-78-9 |
|---|
| IUPAC Name | N-(2-hydroxyethyl)dodecanimidic acid |
|---|
| Traditional Name | N-(2-hydroxyethyl)dodecanimidic acid |
|---|
| SMILES | CCCCCCCCCCCC(O)=NCCO |
|---|
| InChI Identifier | InChI=1S/C14H29NO2/c1-2-3-4-5-6-7-8-9-10-11-14(17)15-12-13-16/h16H,2-13H2,1H3,(H,15,17) |
|---|
| InChI Key | QZXSMBBFBXPQHI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as carboximidic acids. These are organic acids with the general formula RC(=N)-OH (R=H, organic group). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboximidic acids and derivatives |
|---|
| Sub Class | Carboximidic acids |
|---|
| Direct Parent | Carboximidic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidic acid
- Alkanolamine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dl-9150000000-bd052cf3ff1ebe0003e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-9100000000-200ae4caae0046ae7081 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9100000000-29caa4970a6d7f73924c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1290000000-0b3d3474ce54ebe0b49e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01vo-6690000000-b77667810720706b753a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-d946a7611f93c9865774 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 85263 |
|---|
| PubChem Compound ID | 8899 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|