| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 03:13:20 UTC |
|---|
| Update Date | 2016-11-09 01:14:06 UTC |
|---|
| Accession Number | CHEM009916 |
|---|
| Identification |
|---|
| Common Name | Benzenesulfonic acid, 4-[2-[3-[2-(dimethylphenyl)diazenyl]-2,4-dihydroxyphenyl]diazenyl]-, sodium salt (1:1) |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
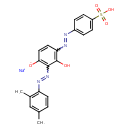
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Sodium 2-[2-(2,4-dimethylphenyl)diazen-1-yl]-3-hydroxy-4-[2-(4-sulfophenyl)diazen-1-yl]benzen-1-olic acid | Generator | | Sodium 2-[2-(2,4-dimethylphenyl)diazen-1-yl]-3-hydroxy-4-[2-(4-sulphophenyl)diazen-1-yl]benzen-1-olate | Generator | | Sodium 2-[2-(2,4-dimethylphenyl)diazen-1-yl]-3-hydroxy-4-[2-(4-sulphophenyl)diazen-1-yl]benzen-1-olic acid | Generator |
|
|---|
| Chemical Formula | C20H17N4NaO5S |
|---|
| Average Molecular Mass | 448.430 g/mol |
|---|
| Monoisotopic Mass | 448.082 g/mol |
|---|
| CAS Registry Number | 1320-07-6 |
|---|
| IUPAC Name | sodium 2-[2-(2,4-dimethylphenyl)diazen-1-yl]-3-hydroxy-4-[2-(4-sulfophenyl)diazen-1-yl]benzen-1-olate |
|---|
| Traditional Name | sodium 2-[2-(2,4-dimethylphenyl)diazen-1-yl]-3-hydroxy-4-[2-(4-sulfophenyl)diazen-1-yl]benzenolate |
|---|
| SMILES | [Na+].CC1=CC(C)=C(C=C1)N=NC1=C([O-])C=CC(N=NC2=CC=C(C=C2)S(O)(=O)=O)=C1O |
|---|
| InChI Identifier | InChI=1S/C20H18N4O5S.Na/c1-12-3-8-16(13(2)11-12)22-24-19-18(25)10-9-17(20(19)26)23-21-14-4-6-15(7-5-14)30(27,28)29;/h3-11,25-26H,1-2H3,(H,27,28,29);/q;+1/p-1 |
|---|
| InChI Key | FIKBURFLPVUDAX-UHFFFAOYSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as azobenzenes. These are organonitrogen aromatic compounds that contain a central azo group, where each nitrogen atom is conjugated to a benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azobenzenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Azobenzenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Azobenzene
- Benzenesulfonate
- Arylsulfonic acid or derivatives
- 1-sulfo,2-unsubstituted aromatic compound
- Benzenesulfonyl group
- Resorcinol
- Xylene
- M-xylene
- Phenol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Benzenoid
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Organosulfonic acid
- Sulfonyl
- Azo compound
- Organic alkali metal salt
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organic salt
- Organic sodium salt
- Organic oxide
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic nitrogen compound
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-0754900000-aa7262d7bf07996ce4cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0972100000-0e958dfa018147c8de61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-4910000000-d0df9a0e504b4809beac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-006t-0902700000-80e6d380c9119c5802d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ba-2933400000-bb0b40f3d9192d9f5f53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-6900000000-7312444c4fe028b9fe9c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 136675507 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|