| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 03:09:10 UTC |
|---|
| Update Date | 2016-11-09 01:14:03 UTC |
|---|
| Accession Number | CHEM009672 |
|---|
| Identification |
|---|
| Common Name | Copper, [C-chloro-29H,31H-phthalocyaninato(2-)-.kappa.N29,.kappa.N30,.kappa.N31,.kappa.N32]- |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
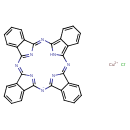
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C32H17ClCuN8 |
|---|
| Average Molecular Mass | 612.540 g/mol |
|---|
| Monoisotopic Mass | 611.056 g/mol |
|---|
| CAS Registry Number | 12239-87-1 |
|---|
| IUPAC Name | copper(2+) ion 2,11,20,29,37,38,39,40-octaazanonacyclo[28.6.1.1³,¹⁰.1¹²,¹⁹.1²¹,²⁸.0⁴,⁹.0¹³,¹⁸.0²²,²⁷.0³¹,³⁶]tetraconta-1(37),3(40),4,6,8,10,12,14,16,18,20,22,24,26,28(38),29,31,33,35-nonadecaen-2-ide chloride |
|---|
| Traditional Name | copper(2+) ion 2,11,20,29,37,38,39,40-octaazanonacyclo[28.6.1.1³,¹⁰.1¹²,¹⁹.1²¹,²⁸.0⁴,⁹.0¹³,¹⁸.0²²,²⁷.0³¹,³⁶]tetraconta-1(37),3(40),4,6,8,10,12,14,16,18,20,22,24,26,28(38),29,31,33,35-nonadecaen-2-ide chloride |
|---|
| SMILES | [Cl-].[Cu++].N1C2=C3C=CC=CC3=C1\N=C1/N=C(/N=C3\N=C([N-]C4=N\C(=N/2)C2=CC=CC=C42)C2=CC=CC=C32)C2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C32H17N8.ClH.Cu/c1-2-10-18-17(9-1)25-33-26(18)38-28-21-13-5-6-14-22(21)30(35-28)40-32-24-16-8-7-15-23(24)31(36-32)39-29-20-12-4-3-11-19(20)27(34-29)37-25;;/h1-16H,(H-,33,34,35,36,37,38,39,40);1H;/q-1;;+2/p-1 |
|---|
| InChI Key | UJBLOYBUFAHAKQ-UHFFFAOYSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phthalocyanines. These are cyclic tetrapyrroles that contain a phthalocyanine skeleton, which consists of four isoindole-type units, with the connecting carbon atoms in the macrocycle replaced by nitrogen. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Phthalocyanines |
|---|
| Direct Parent | Phthalocyanines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phthalocyanine skeleton
- Isoindole or derivatives
- Isoindole
- Benzenoid
- Heteroaromatic compound
- Pyrrole
- Organic metal halide
- Azacycle
- Organic metal salt
- Organic transition metal salt
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organic copper salt
- Organic salt
- Organic zwitterion
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000009000-8c750475c27956e04938 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000009000-74c88278dbd91868d9bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9000125000-b8ccbf9f1f3c3fb709cc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000009000-3f1736e2f53a11f3258b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0000019000-5328d31973c9ee9130f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03e9-0000096000-e95165381ea0d577b52b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 56846440 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|